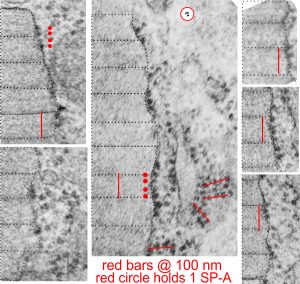
There are several samples of the banding periods and growing end ribosomes, along with a tangential portion showing a grid arrangement of ribosomes on what I presume is an RER profile that contains an abundance of surfactant protein A. The relative sizes marked in black dotted lines are the intervals seen in each separate period of the bands (visible either as 3, 5, 7, or sometimes 9 separate bands) and a bar marker in red (which is the approximate equivalent of the total period (100 nm) and a sample from the internet of a shadow cast molecule of surfactant protein A — in a tiny circle (red circle, white background) which was estimated by the authors to be about 20 nm, Relative to the width of the micrographs which I have used in this image, the single surfactant molecule is sized accordingly. Because the surfactant A protein was shadow cast it appears more defined that would be seen in reality.
Category Archives: Layered intracisternal protein granules in mammalian lung
3 ribosomes – per period, four for a complete band
I used an image with a tangential section on the growing end of the protein found in some alveolar type II cells, where clearly the ribosomes were aligned (not in spirals) in a grid. Using the pretty standard measurement of 100 nm for the distance between major dense bands in the periodicity found in these protein aggregations within the RER, it seems that in a complete period, dense to dense band there are four ribosomes (4 ribosomes per 100 nm) and if one calculates the continuous stacking of these bands (rather than having each period bound individually by RER membranes) then it becomes 3 ribosomes. The number of ribosomes found in routine sections along the leading edge of protein production is approximately 1.7/period (when counting hundreds of sites, and two species of mammal). The count just in this particular image along the leading edge is @1.6 ribosomes, while measured from the tangential grid of ribosomes, it is 4 per 100 nm.
Images from ferret alveolar type II cells give a little more information about the ribosomal organization, as they are more regular and banding is more easily seen, profiles are more orderly, whereas images from guinea pig intracisternal bodies are more often curved, with the banding crossing, or being circular and disorderly, and also there are larger composites of banding patterns. In mongrel dogs… there is typically a single period with 7 bands…. (in some sense the dog intracisternal bodies resemble a little bit… the birbeck granules in Langerhans cells). Here is the image used for these numbers.
(red lines are @ 100 nm, centered over contiguous ribosomes, the 100 nm length was calculated from the mean of the proximate banding patterns, shown in dotted lines)
Maybe only 2 ribosomes per period at the growing end of intracisternal bodies?
I found another set of very organized (grid style) ribosomes at the growing end of what is likely a profile of RER which contains the layered protein I think might be surfactant protein A (in over-production). More data are being gathered. Here is yesterdays video with the second tangential set of a dozen or more ribosomes added.
Since the size of surfactant protein A is about half that of the proposed width of a period (the 3, 5, and 7 sometimes prominent layers that general run parallel to the long axis of the RER cisterna (but not always) it would be really convenient to think that just on either side of the dense band there is a ribosome that is producing the protein. That would be too tidy for words.
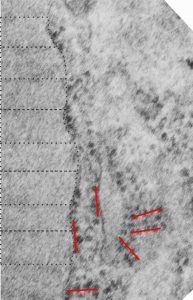
Three ribosomes @ every 100 nm or just 2?
Here is a tangential view of the end of an intracisternal body in a type II cell of a ferret which shows, opportunely, an area of about 45 ribosomes (which would be producing the protein within the RER I am calling an intracisternal body, or a granule. This is an educated guess, since what is behind or in front of the tangential is not really visible, but can be assumed. I am quite certain the original block for transmission electron microscopy has been cut past this field. The grid overlay in this animation shows the spacing of about 3 ribosomes @ 100 nm. This is not a complete match for the number of ribosomes I had counted on the growing end of an intracisternal body, which was inbetween 2 – 4, 2 for an incidentally cut period, and 4 for the most opportune.
What is amazing is the regularity of their assembly here, and their regularity in size.
Protein organization within RER of type II alveolar cells – mongrel dog
Decades (literally) ago when I began trying to figure out what this organized protein was in type Ii cells, I received some peripheral canine lung tissue from from a researcher whose name I actually don’t remember. Several blocks were processed as for routine transmission electron microscopy and occasionally a type II cell would show cisternae of RER which had a protein that was organized in layers similarly to the intracisternal bodies in ferret and guinea pig. Picture is below. Intracisternal space with paracrystalline protein organized parallel to the long axis of the RER membrane, cyan; funny organization of something (too small to be ribosomes) at the end on the left, green; lamellar bodies, gold; ribosomes, purple. No nuclear or mitochondrial profiles are in this cropped image of a type II alveolar cell.
Is there a periodicity to the central band (of 7 or 9) found in guinea pig type II pneumocytes?
I am still working to determine the nature of the protein inclusion in the rough endoplasmic reticulum of some type II pneumocytes (alveolar type II cells) in guinea pigs from an experimental series that was conducted in the 1980s testing whether toluene diisocyanate was harmful for human health. These protein inclusions, I am hoping are going to turn out to be indicators of overproduction of surfactant protein A, or maybe D, though less likely SP-D, because SP-A is the most abundant surfactant protein in lung according to many reports). The protein itself, appears to be so organized I have called paracrystalline, and has not to my knowledge been described in detail, save for a couple of brief descriptions in the early 1980’s literature.
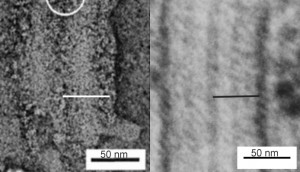
The protein displays several bands, typically parallel to the long axis of the rough endoplasmic reticulum membrane boundaries, which I have shown before, but in this short video clip I have examined the possibility of their being a periodicity to the protein, in the parallel axis to the membranes of the RER, by superimposing five images, removing the light (background) areas, and increasing contrast in the dark areas.
If one accepts that the distance from one dark band to the other (in this case the plasmalemma) is 100 nm, then I think there is a relatively obvious periodicity of about 4 dense areas within the central band (middle between two dark bands), that is, within about the same 100 nm length.
Composite of four frames from tubular myelin (from a transmission electron micrograph found online)
I am searching for the orientation of SP-A within the structure of tubular myelin, not for that purpose really but actually to figure out whether it is SP-A or SP-D that accumulates as a highly organized protein in the cisternae of the RER in type Ii cells of the peripheral lung. Four composites from readily available images of tubular myelin found online show an angular arrangement of the 6 trimers in the octadecamer proposed to be surfactant protein A.
Actually these four particular images come from a paper by Mary Williams, and the micrograph was saved as a screen print, pixels inflated, pasted into photoshop, and 4 individual lattice structures were cut and skewed to fit more-or-less together, their grey and dark and white portions aligned as in a “square” tubular myelin lattice- without changing any of the actual structural data. With the select tool, white and light grey was removed from each layer and transparency adjusted. Using corelDRAW the transparent images were centered, and exported in register, along with an approximate diagram of the positions of the carbohydrate recognition domains (6 surfactant protein A groups (18 CDRs total – so 3 were lumped into one circular object) superimposed. Diagram is seen as a dotted lines at the beginning of the video, and solid circles at the end of the video (no neck or carbohydrate portions of surfactant protein A were drawn in this case diagram). Video was created in Swishmax 4.
Guinea pig type II alveolar cell: pseudocolor transmission electron micrograph
Type II alveolar pneumocyte here pseudocolored as follows: nucleus, purple; nuclear pores light green; perichromatin granules, violet-red; intracisternal bodies, even in the perinuclear space, light blue (these are looking more and more like they might be over production of Surfactant protein A – a collectin which has modest surfactant properties in the alveolar space but probably greater impact on pulmonary immune function. Interestingly it lines up pretty much on the button with two mirror sets of the actual surfactant protein A molecule (which is a group of 18 similar single protein units spanning about 50 nm, so two top to bottom would be about 100 nm the width of one set of bands in an intracisternal body — at least I hope it works out that way); lamellar bodies, of which there are four, gold; and a single mitochondrion, green.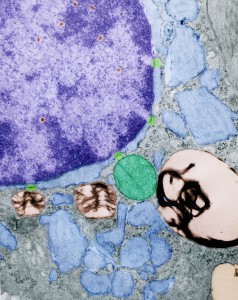
Matching up the banding in electron micrographs and lipid bilayer sheets (possibly SP-A)
Seven inner bands – two outer bands for intracisternal paracrystalline protein in some alveolar type II cells
Here is a short video of the intracisternal protein organization that I have found in guinea pig (and a couple other mammals) in the alveolar type II pneumocytes using transmission electron microscopy. It seems that in the most advantageous photographs, and with the method of preservation that was used on these tissues (glutaraldehyde – paraformaldehyde fix, with osmium tetroxide post fix, either Epon 812 or Spurr’s resin and lead and uranium staining of the thin sections) show a total of 9 bands (inclusive of side to side) a central more dense band which has on either side each, 3 thin bands. The central band has a globular look to it, at least in the way these proteins have been fixed with this method.
Here is the video URL: