I know his is a silly post…. but i bet that actin filaments (generally regarded to be about 7nm in diameter), and intermediate filaments (generally regarded to be about 10nm) and microtubules (generally regarded as something between 20-24nm) really are really a set of prime numbers. Not in sense of being precisely that number of nanometers, … but in the awesomeness of as yet unknown coding in DNA structures with prime number-like uniquenesses, pseudorandom, …which just happens to show up to microscopists in unique circumstances, such as the cytoskeletal elements, that show up as “sets” of PRIME NUMBERs.
Category Archives: Ultimate order, the cell
Desmoplakin KO desmosomes
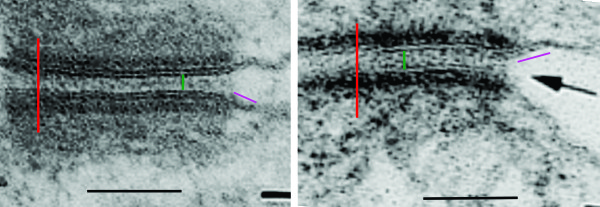
Here is an article by Gallicano et al, which I think has some interesting ultrastructure. They created a desmoplakin ko, and followed some of the changes in structure, but I think there might be more changes than they allude to. I took two of their micrographs and measured (using their own nm markers) the height of the wt desmosomal plaque, the extracellular space and the ring dimension of the annulus… and i think there is an effect of the lack of desmoplakin on how narrow the extracellular space is, and also there is an absence of the very electron lucent layer just above the plasmalemma of the desmosome (just immediately intracellular, almost one could say, beneath the plakophilin and plakoglobin). Since the position of both those proteins as well as the intracellular portion of desmocollin and desmoglein are linked directly and/or indirectly, then it would be reasonable to think that the extracellular dimension (as well as the intracellular thickness of the outer and inner plaques as well as the whole dimension of the desmosome might change.
Extracellular dimension of the ko desmosome, 19nm (according to their own micron marker), and the extracellular dimension for the wt desmosome is ,pre ;ole 16nm. In both the wt case, the extracellular space between those adjacent cells is wider than what is found actually at the desmosome. This is not that apparent for the ko. Similarly the annulus protein seems to be longer (wt=26nm ko=35nm)(though tangential section could easily account for this). Just interesting to see how subtle changes in the TEM are present. Red and black vertical and horizontal bars=100nm. Green bar=extracellular space at desmosome. Pink bar=desmosomal annulus. Loss of IFs in image to the right, reflecting lack of desmoplakin connections with intermediate filaments, and I think too changing the position, orientation, and closeness of the four+ other proteins comprising the desmosome. (mouse). Looking again at these micrographs, they are most assuredly NOT the same magnification as was indicated on their micrographs.
Desmosomal diagram critique: 1
TOPIC: desmosomal diagrams
BACKGROUND: Desmosomes are cell-cell adhesion spots (not spot welds, but more like removable rivets, or bolts with phylanges, meshed with extracellular velcro-like (Ca dependent) intercellular hooks. Desmosomes are very specialized and highly ordered areas that incorporate many of these types of commercial connection devices. Velcro=desmocollins,desmogleins, double headed bolts or rivets=desmoplakin connectivity with the outer dense plaque proteins, and lock washers in particular the plakoglobin and plakophilin portions of the outer plaque proteins. Desmosomes are commonly found in epithelialia, and I have seen them in particular right at the borders of the bile canaliculi in liver, at the apex of gut epithelial cells, and in kidney, and thyroid. I don’t think they are plentiful in lung, but I have not specifically searched for them there. Of course desmosome-like junctions in heart, and muscle cells are unique adaptations of desmosomes. Others researchers have found them in thymus, cornea, and the central nervous system. Desmosomal protein isoforms and connections with filament proteins understandably are different in each cell type, and even with maturation state and location within the same tissue (e.g. different levels of epidermis). Electron microscopy (with electron tomography) remains the gold standard for studying desmosomes. Finding a good diagram of the desmosomal complex, identification of the desmosomal proteins and visual correlations with electron micrographs is not easy. HENCE the following post.
COMMENTS on one diagram: I realize it is always risky to critique a diagram when on has not made a diagram for counter-critique. So please understand this is not a hostile critique, but a learning critique… it is the way I reinforce what is reasonable science and what is regarded generally as “fake data” (ha ha… no deliberate reference to the current state of government and news reporting is made), it is instead an honest attempt to come up with something which is informative, and accurate. The source of the diagram is irrelevant, since the purpose is to examine the content, not to criticize. I will just list the issues i have in bullet format.
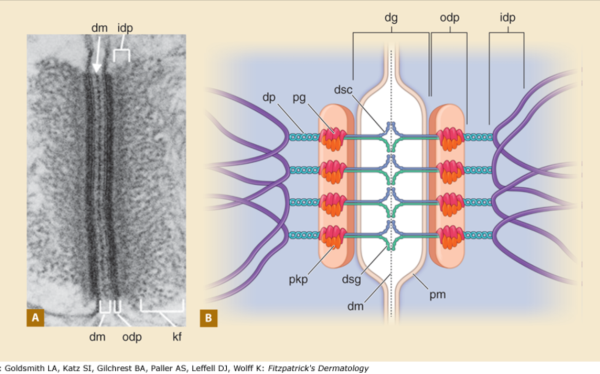
1) the area marked dg, desmoglein, includes the plasmalemma from both adjacent cells…. this probably is not accurate, I would not have commented had the bracket stopped at the outer leafelet of each plasmalemma.
2) dsg and dsc, desmocollin and desmoglein dont really connect in their N terminals as pictured here…. but seem (if one examines the electron microscopy) to be staggered. Even the staggered relationships between cell 1 and cell 2 in their own micrograph have the densities staggered (or alternating).
3) I havn’t seen any reports that say the desmocollin and desmoglein run as a single unit together in the intercellular space which is shown in this diagram.
4) given that desmocollin and desmoglein have a single-pass transmembrane domain and an extended portion into the intracellular space (in the outer and possible the inner dense plaques) it would have been nice to see a little bit of that passing through the red and orange twists that they have labeled pkp and pg = plakophilin and plakoglobin.
5) Since desmoglein does extend into the intracellular space and in this diagram is colored blue, it might have been a better idea to color the dp = desmoplakin some other color, since the continuity of those two molecules as the same color is confusing.
6) it is not tough to make things in relative ratios — a kind of internal scale and when something like the plasmalemma in the diagram is so tiny compared to the other drawn blobs, somehow it speaks to “inattentiveness” or lack of real perception than the need to fit a publication dimension or pixel level. AND, it provides bad data to those who look at the diagram and learn visually.
7) it is actually kind of cute, the common copying mistake that diagrammers make when they try to depict something that someone else has committed an error on. This particular error is soooo obnoxious that it makes me wonder if the scientists who requested the diagram know any more than the diagrammers who diagrammed the diagram. The intermediate filaments in their own micrograph (left part of the image) are not these hairpin wired things darting in to connect with the desmoplakin molecules but they are seen as dots, or pretty much cross sections with a few that look like arcs. Their diagram shows hairpin curved lines (purple) as intermediate filaments as they connect with desmoplakin but shows NONE of the intermediate filaments in cross section, ha ha, but 100% as arcs… this is just an error.
8) the periodicity of the outer dense plaque proteins (that would be a combination of desmoglein, desmocollin, plakophilin and plakoglobin and whatever portion od desmoplakin sticks in to connect as well is not defined in their electron micrograph. I do think one exists, and it has been reported to be the same dimensions (same number of nm between densities), but certainly can’t be borne out in their own TEM.
9) the central dense line (extracellular) where desmocollin and desmoglein attach, in the diagram they present would surely show a lucent dot, where they have their molecules coming together… NOT as is typically seen, a definite density, and regular periodicity.
10) The desmoplakin twisted glue lines has two prominent areas at the N and C terminals which some have depicted as
So this if fun for me, again, not to aggravate anyone, but to identify which diagrams can be used to educate, and which need to be pitched as fake news.
Best desmosomal model so far, but still incomplete
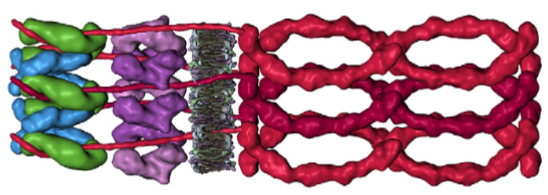
“The cytoplasmic surface of intercellular junctions is a complex network of molecular interactions that link the extracellular region of the desmosomal cadherins with the cytoskeletal intermediate filaments. Although 3D structures of the major plaque components are known, the overall architecture remains unknown.” Al Amoudi et al, PNAS April 2011.
Theirs is the best diagram of the desmosome that I have found to date.
1) obvious lattice structure (periodicity)
2) intracellular lattice pretty much matches the periodicity of the extracellular lattice
“No intracellular molecular model for Dsc or Dsg “The structures of the cytoplasmic domains of desmoglein (Dsg) and desmocollin (Dsc) are not available. However, an alignment of their sequences with those of E-cadherin shows that most of the observed E-cadherin/β -catenin interactions are likely to be conserved in desmosomal cadherin/plakoglobin complexes. Therefore, we modeled plakoglobin-binding residues of desmosomal cadherins on the E-cadherin/β-catenin structure.” “Most of the cytoplasmic domain of Dscs (approximately 150 among approximately 200 aa) is modeled based on the E-cadherin/ β-catenin structure. The cytoplasmic region of Dsg is significantly larger and cannot be accounted for in our model. We speculate that the remaining region of the cytoplasmic domains of Dsg could span the region between the ODP and IDP, as proposed from sequence analysis (23), and thus it is not shown in our map of the ODP. According to Al Amoudi. Giving all credit to them, here is their diagram…. which needs some work, i know they know. Firstly they have made the intracellular and transmembrane portions of the moleules (Dsc and Dsg) the same, which they say are not the same. Secondly… i dont think the figure 8 shape is what is seen in TEMs, sometimes yes, but mostly no… so the orientation of these molecules needs some adjusting. Thirdly… the inner and outer intracytoplasmic dense plaques are not the same relative sizes… but their molecules here are the same size… and for all my observing of models of
Desmoplakin and IF attachments
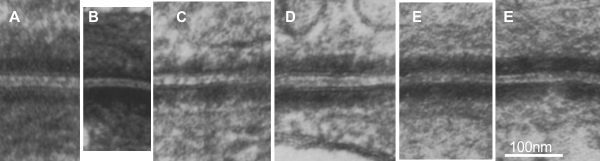
I tried to figure out the transmission electron micrographs of an article on desmoplakin and intermediate filament attachment in cultured cells by Bornslaeger et al, The Journal of Cell Biology, Volume 134, Number 4, August 1996 985-1001 and really found the descriptions and TEMs difficult to interpret. Not diminishing their efforts, the micrograph B is clearly not the same magnification as the others which were all pinned under their micron marker of 100nm. Secondly they point to some strange thing as the IF in their first and second images (not shown in what i cropped as the left (A and B) images when there are IF that could have been pointed out just above the inner dense plaque. The IFs which are not connected to the inner dense plaque (they attribute to the loss of functional desmoplakin IF connections) are easy to see in E and E (both from their figure E, i cut and pasted two portions from their figure). I guess they accomplished what they set out to acomplish… the diminution of the inner plaque and loss of densities beside-with-attached-to the adjacent IFs. I sure didnt enjoy reading this overly acronymish publication (haha). Desmoplakin is an abundant molecule in desmosome formation, and accounts for some serious electron density in the inner plaque region. It is interesting, too, that the IFs are parallel to the plasmalemma, regardless of the blocking of functional desmoplakin, this orientation did not change from what is typically seen.
Insect septate desmosomes.
Michael Locke wrote a paper (rather a brief note) on the structure of septate desmosomes of a caterpillar (Calpodes ethlius). LINK HERE. It shows a possible arrangement of such a structure, honeycombed in one tangential array and in other cuts showing a parallel periodicity. The maganification bars on micrographs are not specific, i have to determine that they represent about 80nm, as remeasured using the size of an insect ribosome as the gold standard. I redrew a diagram (I am not sure why an 16 pronged asterisk like symbol was chosen for his diagram but it is reproduced and edited and overlies a portion of his micrograph of a tangential section of a septate desmosome. Another image from the same micrograph (enlarged to the same degree) has one potential that he recommended for the parallel section of the septate desmosomal honeycomb cells. His bar marker and the ribosome size are added to the images below. A larger scale diagram (slightly modified (with different planes of section that he provided) is included (red asterisk-like structures on the right lower portion of the png. His dimensions of adjacent plasmalemma being 14nm apart dont fit the size in the same micrographs of a typical ribosome. septa are more like 25-30nm lucent area and 40nm dark to dark line. The diameter of one hexagonal structure he named at about 105nm.
 Just googling what the cadherins might look like in insects and other “bilateral organisms” using the term in the introduction of Sasaki et al, 2017 (LINK HERE) looking at those protein structures, correlating the differences in the extracellular EC repeats, might easily explain the differences in dimensions and organizations of these sepatae (and other) desmosomes.
Just googling what the cadherins might look like in insects and other “bilateral organisms” using the term in the introduction of Sasaki et al, 2017 (LINK HERE) looking at those protein structures, correlating the differences in the extracellular EC repeats, might easily explain the differences in dimensions and organizations of these sepatae (and other) desmosomes.
ONE NOTE: it is clear that there are restricted positions where desmosomes in the mammalian liver are found…. mainly at the junctions of hepatocytes at the point they form the bile canaliculus…. reading from a review by John Pettitt (LINK HERE) he mentions that the classical cadherins are responsible in part for cell polarity.
JUST a note: in the lineup of evolution, it looks like the transmembrane, and intracellular and the very close extracellular (right next to the transmembrane domain) dont change very much, compared to the pile of extracellular repeats and other kinks.
It is an awesome universe. Life is awesome and complicated. Learning about life is worth the effort required to maintain sanity.
Moth desmosomes – nice differences from mammalian desmosomes
I found this paper by Doreen Ashhurst on insect desmosomes which had some transmission electron microscopy of moth desmosomes which are clearly very different than those found in mammals. Brief notes. JCB 46: 421-425, 1970. It is a scanned pdf so the images are not that well preserved but from the text and images here is a set of general similarities and dissimilarities between moth and mammalian desmosomes (typical epithelial cell connections). The list begins at the most intracellular zone to intercellular space, beginning with the cytoplasmic structural proteins, the plaque itself (the outer dense plaque and inner dense plaque in mammals), the plasmalemma, and the central dense line where the cadherins hook up (at least there is a central dense line in mammalian desmosomes, seemingly not in moth desmosomes) .
| WAX MOTH | MAMMALIAN |
| 600nm | 250-300nm diam |
| oval shape | pretty much round |
| cytoskeletal protein=microtubules | cytoskeletal protein=intermediate filaments |
| MT parallel to desmosome | IF parallel to desmosome |
| lucent area by microtubules | desmoplakin molecules by IF |
| fuzzy outer plaque w periodicity | neat tidy outer plaque w periodicity |
| periodicity about 20nm | periodicity about 4nm apart |
| intercellular space about 20nm | intercellular space at desmosome @10nm |
| desmosome has an annulus | desmosome has an annulus |
| intercellular densities periodic | intercellular central dense line |
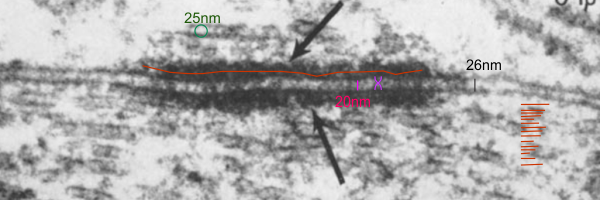
One thing seems true, the periodicities are less marked in insect (thought the X and Y configurations of what would be equivalent to the desmogleins and desmocollins are just barely seen in this photo by Ashhurst I bet they do have some relationships to mammalian desmosomes. The differning dimensions of the desmosomes between moth and mammal are really quite interesting.
Her micrograph did not have a scale bar but I used a microtubule (with a diameter of 25nm) as the standard. Red line through desmosomal outer plaque used to measure the diameter in the long dimension (presumably) of the insect desmosome. Microtubule in the upper left used to get an estimate of overall magnification of the image (microtubule at a nominal 25nm diameter). Similarly, moth to mammal, there was a slightly reduced width (height) of the intercellular space at the central part of the desmosome, than there was at the extracellular space between the adjacent cells at the points away from the desmosome. The distance within the desmosome being smaller than the distance (height or width of the intercellular space) adjacent but which is NOT part of the desmosome. There is an annulus for the moth desmosome, just like for the mammalian desmosome.
I love that this author says…. “intermediate filaments may run parallel to the plasmalemma in mammalian desmosomes…. ” i know she saw that problem with the early interpretation…. thats fun, and she saw it way back in 1970, and the same is misjudged today. Old habits die hard, and hardly die at all.
Classical and desmosomal cadherins: bullets
Surface proteins
Nidi for cell aggregation
Influence tissue states (layering)
Mediate selective cell-cell adhesion
Regulate embryologic cell sorting
Switches specific cadherin expression
Segregates embryological layers
Image from Farquhar and Palade below, and enlargement
It looks to me like the extracellular domains of the desmosomal cadherins (model found from some other publication obviously) fits well below the plasmalemma of the two apposed cells. This puts the transmembrane and intracellular domains of the cadherins here as a huge portion of the remaining molecule to span from the top and bottom of the punctuated extracellular space (this doesn’t include the dotted central density of the extracellular space generally called EC1) quite a ways from the plasmalemma. I am waiting to find a WHOLE molecular model of desmocollin 2 or desmoglein so i can visually “fit” those molecules THROUGH the plasmalemma on both sides of the cell-cell junction. The red circles, likely the densities at regular intervals of the connection of the intracellular portions of desmocollins/desmogleins with the plakoglobin and plakophilin? There are some very nice tomographic images and studies of desmosomes, but they seem to fit the TEM of the extracellular molecules pretty well (not perfectly) but dont manage to show the transmembrane and intracellular portions of the cadherins with any clarity, in my opinion. And I cant find any molecular images of the whole cadherins (all 3 domains).

More images of desmosomes mostly hepatocytes but many species, many double tethers with mitochondria
The desmosome is not a “spot weld” and not a “bolt” but maybe a modified? “rivet”
A modified rivet
There are many names which have been given to these adhesion points, that is the desmosomes. They occur as spots, yes, of about 200-500nm in diameter, but they are certainly not spot”welds” which would imply that the two cell membranes involved become ONE, ie, the surfaces of the two sides being joined are changed. (This is more reminiscent of the tight junction, matching the spot-weld description somewhat but totally unlike a desmosome).
Others have called the desmosome a BOLT. This isn’t accurate either because the central line in the extracellular space of the desmosme is more like a velcro joint and not a solid inflexible mass but it is a moveable collection of various assemblies of cadherins – mainly desmocollins and desmogleins) where the center portion of the juncture (where the cadherins are bound in a central dense line with a distinct periodicity (something around 6-8nm) can be made and broken depending on ion concentration and cell signaling. This is not at all like a solid bolt-like structure, instead it is more like a zipper. Presumably the bolt analogy which referrers to a something “imagined” but not seen with electron microscopy was just a careless comment since a desmosome penetrating both sides of two adjacent cells is certainly NOT SOLID. A bolt is not only solid but is temporary (or can be temporary) this on the other hand is more like a desmosome.
The desmosome is made up of hundreds of molecules positioned parallel and perpendicular to the plasmamembrane, allowing for flexibility and resistance to various stresses: shear, cleavage, tensile and peel, compressive and torsion. I am sure there are others. The desmosome might be a partial solution to shear, but it would be single shear, since there are only two surfaces involved, one from each adjacent cell.
and ensuring the adhesive molecules penetrate the proximate side of the adjacent plasmalemma of both nearby cells. The bolt analogy doesn’t work since the central joint of the desmosome is easily “made” and “broken” depending upon cell signaling, stage of cell cycle, calcium concentration and sensibility of the cadherin molecules, ie. whether the desmosome is going to be removed or substituted, or unzipped.
- semi-permanent
- cylindrical shank with “head” within each of the two adjacent cells
- similar to a buck at the intercellular junction between the cadherins from each of the two cells.
- major differences is that the “buck” is two sided, like the velcro…. and the integrity of the attachment sis maintained by calcium concentration (and likely many other as yet unknown factors).
A rivet however is defined as a “permanent fastener” so here we have a problem right away…. desmosomes are made and unmade on a regular basis, moved from here and reassembled there to accomodate changes in, activity, stage of the cell cycle, size, forces, maturity of the cells, and countless others. In fact all junctions have to be totally responsive to intra and extracellular events.
Is a desmosome a little like a pop nut, or hollow wall hanger, where the is flexibility in the tethering of cadherins…. just a little, and also in tethering the intermediate filaments by desmoplakin?
Or is the desmosome is more like a bilateral (two headed) unzippable ductile lap rivet.
One of the unique features of attaching two cells with a desmosome that has no parallel in bringing two pieces together in a rivet, is that both sides in the rivet process need to be accessed….which in building….can present a problem. However, intracellular activity makes this access routine and the intercellular components are managed from the inside of each of the respective cells.
I have thought for a while about the arrangement of molecules, and its very nice parallel+perpendicular+parallel+perpendicular line up and felt that this was certainly better than any man-made adhesion rivets…. I wondered whether such a complex set of parts could actually be developed into a real live-functional construction friendly rivet.
Brazing or soldering joint….not applicable in my opinion.
Types of joints….. the desmosome is NOT a butt joint, clearly a lap joint. The spacing of desmosomes just like rivets, will be dependent upon many biological parameters, including states of differentiation.