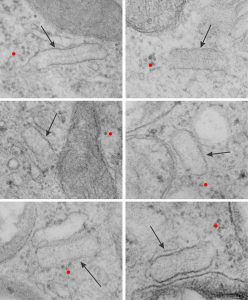
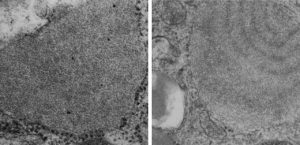
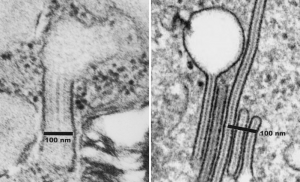
More evidence that SP-A bouquets are found in tangential sections of the protein filled bodies found in some alveolar type II cells. I am pretty convinced that these bodies are for the greater part surfactant protein A that takes the form of an highly organized (layered) structure that shows linear organization and period repeats that are consistent with an end to end configuration of surfactant protein A, again mirrored. That is, bouquet end to tail end, tail end to bouquet (times 2) per 100 nm period with the central and outside lines of the periodicity representing the carbohydrate recognition domains.
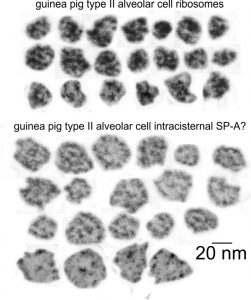
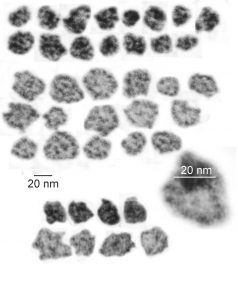
Previous posts show this intracisternal protein on cross section perpendicular to the long axis of the periodicity – or layering, which is easily recognized, and quite orderly, but since the protein inclusions also show curvature and in some cases end to end exchanges in layering direction, and others even as single tubules on cross section, and there are usually “growing ends” with ribosomes along that growing edge of the RER profile. The number of ribosomes counted out comes to something on the order of 2 ribosomes per period, and also there are occasions (shown on this blog site) where ribosomes and the protein (presumably surfactant protein A) appear in the same configuration that one would expect from a top down view of the SP-A bouquet right adjacent to each other, it just seems that the anatomy and the protein structures fit together.
All these new data were found 35 years after the initial discovery of that structure with TEM and that initial search for what this body was, and which started back in 1980 when I queried a respiratory scholar and pediatrician then at CCHMC — who was/is a surfactant specialist — but who also was not interested. So this study was taken off the back burner in 2016 for a little fun and replay. I am quite sure that this RER compilation of protein represents an overproduction of surfactant protein A which behaves in the RER profile like other collectins (langerin being a great example and even showing layering patterns onto which a langrin molecule image can be placed, though producing a different orientation of periodicity than SP-A, but conceptually similar, and obviously related in the greater scheme of the way collectins are ordered within the RER. Here is a quick animation that shows one such tangential section of an RER profile filled with this protein, but the periodicity is spread by a tangential orientation to the plane of the section. The darker regions of the image relate to the darker regions of the banding and those areas represent the top clusters of trimers, assembled as the 18 mer of SP-A open and with a center dot presumably representing the neck. Thee micrograph was only contrast enhanced and the burn tool used in photoshop, two scratches were edited out with the bandaid tool, otherwise pixels were unchanged from the original micrograph scanned at 3600 pixels per inch. Images imported into CorelDRAW, exported as transparent png images, and the animation was made with Swishmax 4. (no data were changed)
Thin sections here are probably about 100 nm thick, maybe a little more, thus there is overlap between one 18 mer and the other. It has to be determined (but looks plausible) that the hexagonal SP-A stacks up like a honey comb.