I worked hard and lobbied continuously for this cover. Here is a link to the journal in general.
Category Archives: Ultimate order, the cell
Substructure of the lysosomes containing perfluorodecyl iodide
Substructure of the lysosomes containing perfluorodecyl iodide is very interesting. There are issues both with the lysosomal membrane, which in some places may be continuous with other membranes, in particular, i think maybe the RER. Because perfluorocemicals are kind of “slippery” and I think they can travel easily retrograde-style back up into the RER, then it makes sense that some profiles of RER would be seen at sites within the lysosome periphery and the adjacent cytoplasm. I haven’t been able to equivocally find ribosomes on the actual lysosomal membrane to verify that concept. There are perfluorodecyl iodide tiny crystals…. this is interesting…. an obvious type of fracture or separation plane along the long axis of the larger crystal inclusion certainly is evident…. and i am more convinced that the pattern of lysosomes (a dotted or tubular pattern) is on the order of 20-30nm diameter or width.
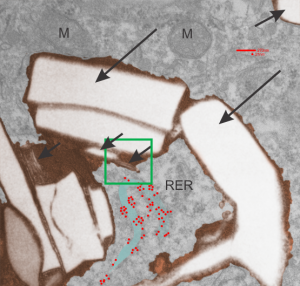
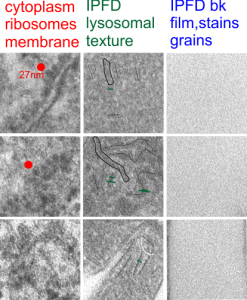
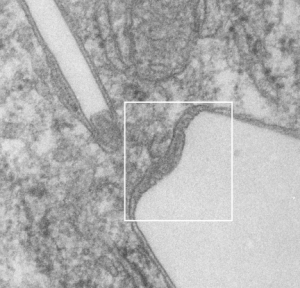
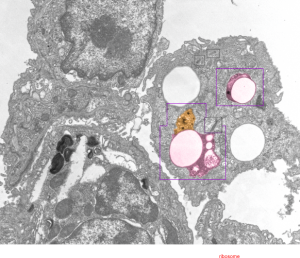
Here is an electron micrograph: mouse, neg 9722_10%IPFD, 5%F68 infused 100cc/kg, 8-14-73 sac 5-8-74, 267 days post infusion; liver. Brown areas=lysosomal enzymes within a bounding membrane, white=IPFD, red dots are ribosomes; pale green is cisterna of RER, green box is area enlarged below.
So the bottom two images, left unretouched, right unretouched but with translucent overlays where I see a pattern within the lysosomal enzyme membrane. Arrows here point to fractured off crystals of IPFD, and the red line is a marker for 27nm which is the same size as the ribosome measured (top inset)…. this makes me wonder if there is some connection between this pattern and lysosomal contents.
Periodicity at ends of IPFD crystals in macrophages
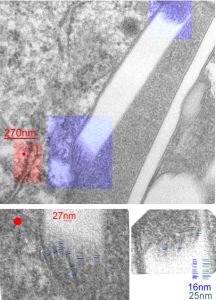
I looks like the lysosomal enzymes at the end of the IPFD crystal inclusion-structures might be tubular. But then as the enzymes pass away from the IPFD itself, there can be a coiling up of the protein with less rigidity. Measurements at ends of IPFD look smaller than those in the lysosom proper… may be error of just having only measured a single crystal. Same negative and block as previous post, and the ends of the IPFD crystal are colored bluish, and the area measure for ribosome size is in red. Enlargements below, with areas specific measured highlighted. The dense part of the period and the lucent areas beside it.
Texture comparisons: background, cytoplasm and IPFD-lysosome
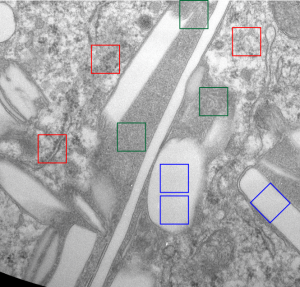
I am trying to determine whether there is a predictable texture to the proteins within phagolysosomes which contain IPFD (perfluorodecyl iodide)(which I am positive there is–so this is academic). I have compared territories from one micrograph with marked inset boxes: red boxes=cytoplasm with a ribosome size marker for comparisons of texture-size); green boxes=portions of the compact and textured lysosomal proteins found just within the limiting membrane of the phagolysosomes which contain IPFD; blue boxes=the background texture of the micrograph found over the “empty” IPFD granules which consists of the acetate film grain and the stains– osmium, uranyl acetate and lead citrate grains. No photographic paper grains are present as this was scanned at 5000 ppi from the negative.
I measured a similar texture pattern from the end-on portion of the lysosomal proteins in last post, and dimensions are similar but not identical… likely my fault, not the fault of the proteins. So todays measurements are something around the following: dense areas at around 17nm (corresponding to the “spot” in yesterdays post which was around 13nm) and the surrounding more electron lucent space measures about 34nm (which in yesterdays post was closer to 40nm)–all likely resulting from variations in how i measured ribosome size, which is the benchmark.
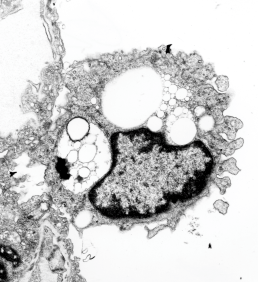
Image below is from a randomly selected phagocyte in the liver (I cannot identify whether this is a Kupffer cell or other phagocytic cell type). These cells can be closely aligned with sinusoids but also near portal tetrads. They are typically “overflowing” with IPFD particles. IPFD stays around for months, unlike other some perfluorochemicals.
Neg 9722 block 3775 10% IPFD 5%F68 injected 8 14 1973 100cc/kg sac 5 8 1974 mouse liver 267 days.
IPFD crystals in lysosomes
RedI cut around the top end of the crystal inclusion in the image from the previous post, and erased (in photoshop) areas that allowed me to pseudocolor the objects that I could see in the lysosomal phase of this phagososome. I think the periodicity is going to be interesting with a 13-15nm round central dense area and a lucent area around that density (like a donut), still with a layer of dense protein or a trilaminar membrane.
It was fairly easy to see the repeating dots and lucent surroundings emerge as a repeating pattern along the terminal membrane at the long axis of the IPFD inclusion. IPFD crystals show for the most part a definite polarity, and any substructure in the IPFD inclusion itself is see on the butt ends of the short width axis, while a very smooth bounding area is seen on both sides running in a parallel direction.
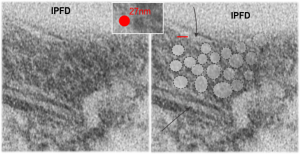
Below, same pix as 9 13 2017 post but with a bounding box which aligns to the inset image below again.
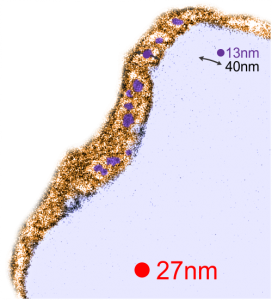
Red dot=ribosome at 27nm diameter, purple dot=central densities, tangential end sections, 40nm dimension for a cross sectional diameter of the electron lucent area surrounding the purple dot, and 13 nm dimension is for the purple dots (some larger some smaller).
CAS names for the iodine containing perfluorochemicals investigated for use as artificial blood
CAS names for the perfluorochemicals investigated for use as artificial blood (blood substitutes) are really important, as so many acronyms appear for the same chemicals. I have to say it has been a major headache just going through notes, and posts, and publications, to figure out the long names and CAS numbers for the acronyms used in the 1970s. I do thank the kind person at Fluorox labs (he might not want his name mentioned? i did not ask him) for some valuable info, and of course being a chemist, he suggested that I only use the CAS names (which i will try to do).
Two perfluorocarbons with at least one iodine atom, were made into emulsions and infused into mice in the 1973-1974 range by Leland Clark Jr (and the research faculty and support in his laboratory) one was called n-PF alkyl ethyl iodide (PFEI for short) and Fluorox Labs gives CAS 68188-12-5 to this compound and a second compound Perfluorodecyl iodide (IPFD) – presumably 1-iodoperfluorodecane) has the number CAS 423 621. I am really curious to see how these differe in vivo, since at the time, no one seemed to “have the time” to look over differences. There were 4 mice total, one mouse gave up several different tissues, from the others, only liver. Details below.
n-perfluoroalkyl ethyl iodide (PFEI) (CAS 68188-12-5)
Blocks 4759-4762 mouse 4, liver (one list says 4759,4760), liver
emulsion# 740313; PFEI 10% F68 5%, infused at 50cc/kg,
infused 3-13-1974 –sac date 1-14-75 (307 days)
perfluoro decyl iodide (IPFD) (CAS 423 621)
emulsion #730814, log#86 IPFD 10% F68 5%, 100cc/kg
2339-2342 mouse 1, liver (no osmium)
Blocks 2343-2346 mouse 1, liver
Blocks 2347-2350 mouse 1, kidney
Blocks 2351-2354 mouse 1, spleen
Blocks 2355-2358 mouse 2, liver (no osmium)
Blocks 2359-2364 mouse 2, liver
emulsion #730814, log#86 IPFD 10% F68 5% 10cc/kg
infused 8-14-73 –sac date 5-8-1974
Blocks 3774-3780 mouse 3, liver
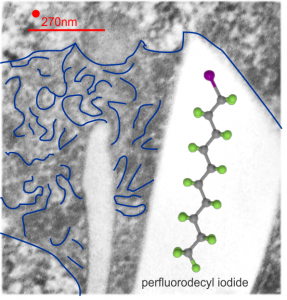
This is what I believe is the structure (inset within the large, presumed, crystalline footprint formed by IPFD; grey carbon atoms, green fluorine atoms and a fuchsia atom being iodine). Thanks to Fluoryx labs and Chemspider and wikipedia and of course google.
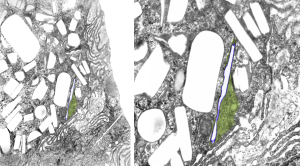
Two white rhomboid spaces where IPFD was once in these lysosomes are seen (vertical spaces–one large one quite thin), and the blue outline is the apparent limiting membrane of the lysosome (which encloses the crystalline IPFD), and the blue lines that wiggle within the lysosome show a sheet-like-periodicity that needs further investigation but is very clearly a pattern in most of the lysosomes with IPFD inclusions, and not really present in lysosomes from the other artificial blood substitutes that I have looked at with TEM (that would be a long list).
Red dot is ribosome to estimate size at 27nm, red line is micron marker derived from ribosome size. No measurements yet made on the longest crystal length, but it is going to be many microns for the largest, and the directionality has yet to be associated with any cytoplasmic elements.
Perfluorodecyliodide in a lysosome
This is an interesting perfluorochemical (IPFD) and in the mid 1970s it was used as an emulsion for studies on artificial blood (blood substitutes). It was the only perfluorochemical used that had a crystalline structure in vivo, and while i have posted previously on it, i have hunted up about 10 negatives (i think the only negatives i have) from those long forgotten files and will examine whether or not there is any peculiarity about the enzymes, perhaps even a periodicity to the lysosomal enzymes which surround the IPFD inclusions. I have to assume that the cell in which the crystals are appearing is either a kupffer cell or an hepatocyte, I can’t be sure which.
Just from the beginning, here is an image scanned at low ppi, but it shows a curly look to the lysosomal structure surrounding this narrow IPFD inclusion. IPFD is purple outline, green is the lysosomal contents. Image on right is enlarged from left.
PP5 (perfluorodecalin) liquid breathing mouse lung for 1 hour with 1.5 hours recovery
PP5 or perfluorodecalin (image to right) was used as a possible component of artificial blood, or called otherwise, blood substitute(s), but never really attained full research or understanding necessary to determine whether it was a safe substitute for blood or not. Some of the early electron microscopy, which would have benefited from a huge influx of money, early on, for microscopists to examine the effects on all cell types, in vivo and in vi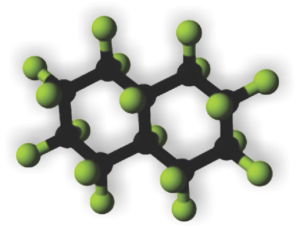 tro, but too little was forthcoming to give it a thorough exam. It was clear however, that macrophages and the immune system were going to be big players, both because of the volume of the chemical that was infused or “breathed” and because of its alleged inert nature but odd surface tension properties.
tro, but too little was forthcoming to give it a thorough exam. It was clear however, that macrophages and the immune system were going to be big players, both because of the volume of the chemical that was infused or “breathed” and because of its alleged inert nature but odd surface tension properties.
In retrospect, looking at tissues which were taken from Leland Clark Jr’s lab in the 1970s, these particles (droplets) behaved strangely, seemingly dependent upon chemical properties, and of all examined ONLY one perfluorochemical had a shape in the cell other than very very round (this was perfluorodecyliodide – looking like a crystal instead of a droplet, as seen in this image from the liver after infusion of an emulsion).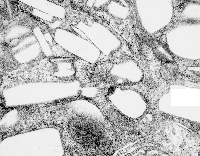
Liquid in the cell in almost always is “slightly out of round” the best examples being fat droplets. They can be oval or indented or squeezed, as well as round.. but perfluorochemical droplets are ROUND. I would think this could be attributed to their heavier than water density.
Another property (mentioned in previous posts) is that they have various capacities to be re-emulsified, at least that is what i call it, within the cell. Example from the liquid breathing mouse posted yesterday (breathing silicone oil vs breathing E2) shows that the respective macrophages respond by producing different quantities, and also likely types of lysosomal enzymes, in addition to the recycling they do of the alveolar contents (that is, in recycling surfactant and other junk in the alveolar space.
So comparing silicone oil, E2 and PP5… seen here, once again, the lysosomal responses are quite different. Silicone oil didn’t encourage lysosomal production (and surfactant recycling) as much as E2, and E2 in turn caused a lesser (though still pretty dramatic) response than PP5. Actually, from notes taken from this series of experiments (Clark never did anything twice which made it really tough to keep notes) those mice breathing PP5 did not fare well, most did not survive as long as those breathing some other of the perfluorochemicals. E2 was probably the best in this regard, just due to the sheer number of samples and micrographs, this is what I remember.
So here is an electron micrograph of an alveolar space from a mouse which breathed PPF for 1 hour and was allowed to recover for 1.5 hours. (1499_5512_PP5_1hr_1.5hr recovery).
 Highly rounded droplets, with very well defined lysosomal enzymes. The two non-highlighted droplets are likely to have (as the upper left already indicates) masses of lysosomal enzymes in a different plane of section. There are some smaller, and also very tiny droplets of PP5 visible in the two highlighted LE/PFC bodies. I did find some dense areas of the ER (not likely RER, but something different) that showed an unique substructure (see the inset with three small sample images (enlarged from boxes in the top figure) showing what I noticed.
Highly rounded droplets, with very well defined lysosomal enzymes. The two non-highlighted droplets are likely to have (as the upper left already indicates) masses of lysosomal enzymes in a different plane of section. There are some smaller, and also very tiny droplets of PP5 visible in the two highlighted LE/PFC bodies. I did find some dense areas of the ER (not likely RER, but something different) that showed an unique substructure (see the inset with three small sample images (enlarged from boxes in the top figure) showing what I noticed.
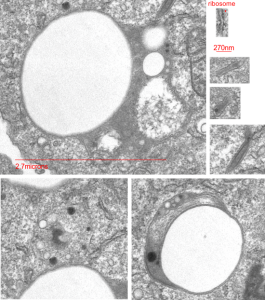
Inset is enlarged and micron marker is determined from the size of an average (27nm) ribosome, and segments of the inset are all at the same enlargement from the original.
Silicone oil liquid breathing, one macrophage
Here is a not-so-hot electron micrograph of an alveolar macrophage, but the inclusions of silicone oil are kind of interesting. By 72 hours after just one hour of breathing silicone oil, show droplet inclusions that look a lot like they are doing the Oswald ripening thing, but also havn’t stimulated quite the lysosomal enzyme thing that macrophages that have phagocytoses perfluorochemicals show. There are also bizarre areas of the lysosomes which are not typical of the PFC inclusions. So differences – noted!
One of the more interesting differences (well two) is that there is almost no discernible trilaminar membrane around the inclusions (LE/SiO droplets) and there is certainly not a double outline (one is the trilaminar membrane of the lysosome, the other the protein coat as seen on PFC droplets (see previous posts)), so those two differences in ultrastructure plus the lack of a definite enzyme overload in those particles in alveolar macrophages that contain silicone oil droplets, and the sloppy separation (or ripening) of the individual droplets of silicone oil vs PFC droplets, makes for nice contrast between the two.
Here on the left is an enlargement of silicone oil inclusions in an alveolar (1 hr breathing 72 hr recovery) macrophage and on the right, E2 inclusions 3 hr breathing 48 hour recovery (biasing the E2 in the direction of less organization, but still showing much more organization and lysosomal enzyme content than the silicone oil).
Protein coat? in phagocytosed E2 inclusions in alveolar macrophages?
The bounding electron dense coat of proteins on the perfluorochemical droplets, whether phagocytosed from a neat liquid as inhaled from liquid breathing, or provided to the blood stream as artificial blood, it seems to be a fixture of the morphology. I have scanned the edge of a single alveolar macrophage for evidence for “point of entry” of a droplet of E2 into said cell. It is pretty clear even from this slightly too thick, thin section of a mouse lung (1349 4840 E2 3hr 48hr recovery) that the electron dense coat around droplets exists in lung of liquid breathing mice before they are phagocytosed by alveolar macrophages. In this case the proteins likely to be lipid molecules derived from surfactant.
I suppose it is also likely that the slight lipophilicity of perfluorocarbons means that the surfactant lipids (such as phosphatidylcholine and phosphatidylglycerol) and the amphipathic proteins in surfactant (like SP-B) might contribute in some way to this droplet coat (as derived from surfactant already in the alveolus) reflecting its contribution shown in vitro studies suggesting an ability uptake of bacteria from the alveolar space (the leap is to E2 droplets) (ref Yang et al below)
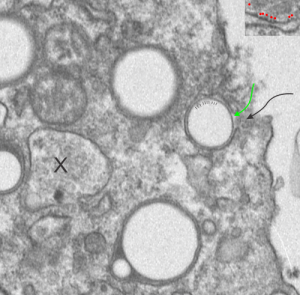
There are at least 4 whole E2 droplets, and two cut off (left hand side of micrograph) droplets of E2 in this image. The tiny dotted periodicity were shown by lines in the droplet at the middle right, the green arrow points to the electron dense bounding layer of “some lipid and protein components from the alveolar space, likely from surfactant”, and the black arrow points to the space in the plasma membrane where the particle is likely being engulfed by the alveolar macrophage. Ribosome size is judged by the upper right corner inset with a portion of RER enlarged identically with the larger micrograph. X marks an inclusion type that I have seen before but do not recognize. Any suggestions from the audience are welcome (millermn – ucmail dot uc dot edu).
Surfactant protein B and lipids look like a good candidates for the electron dense cover for E2 droplets — reading the article (Hawgood et al) and Yang et al specifically referring to SP-B and from which I quote: “In no rank order these activities include membrane binding, membrane lysis, membrane fusion, promotion of lipid adsorption to air–liquid surfaces, stabilization of monomolecular surface films, and respreading of films from collapse phases.”
SP-B has some interesting properties as referenced in many articles in PubMed which suggest that it has saposin like properties. Wikipedia identifies saposins thusly: “Saposins are small lysosomal proteins that serve as activators of various lysosomal lipid-degrading enzymes”. It is interesting that the huge lysosomal response that is induced by liquid breathing both in alveolar macrophages (and also when perfluorochemical emulsions when given IV) might be seen in the types of lysosomal bodies found, the length of time required to degrade/and/or reassociate the enzymes that appear to cover the droplets in the alveolar space, and concentrate (add more? or recycle more? possibly be reflected in the fine granularity of the electron micrographic views of these MVB/LE/PFC organelles. The amount of re-emulsivication of phagocytosed E2 in alveolar macrophages appears to be time dependent, and would fit with the tim required to increase in production of some lysosomal enzymes and become associated with the E2 droplets.