Here is a summary table (already needing to be updated to ferret pix=365; guinea pix=664 with animals being n=61; rat pix=44, n=3: for a new total number of micrographs of 1456), which I could not resist making “pretty”. It does give you an idea of how many electron micrographs have been perused in order to make some statements about whether this protein granule is a part of the regular surfactant machinery or whether it represents something in the way of an “overproduction of protein – hypothetically, surfactant protein A) in a disease state. (I am leaning toward this view). (115 total, 12 species, at least two dozen obscure experimental groups, ages and controls mixed in, three types of processing fixatives, at least three embedding compounds, silver and gold sections, standard uranyl acetate lead citrate staining, and half a dozen different electron microscopes utilized over 30 years, and tissues from at least a dozen different investigators (their materials plus mine). Well over 1000 type II cell images examined in detail.
Category Archives: Layered intracisternal protein granules in mammalian lung
Alveolar type II cell, Dog: granules that might be surfactant protein
Going back over 114 micrographs of alveolar type II cells in the lungs of dogs (n=9; mostly controls from other experiments or ozone treated, taken expressly for lung morphology looking for ICBs) I found several reasonable occurrences which are montaged here. These images are cropped from micrographs of various magnifications, and while I did enhance contrast on some light ones, no dust and scratches or other photoshop applications were used. There are some pretty nasty micrographs here, but this is an unbiased sampling of all the ICBs that I found in all the dog tissues that I had photographed in the 1980s. At least three fixation fluids were used: Modified Karnovsky’s, a glutaraldehyde paraformaldehyde mix, and direct osmium. It seems that the direct osmium preserved the periodicity in the granules better than the aldehydes, but the micrographs were not as informative overall. White arrows point to the granules (ICBs). Only in two micrographs were the granules seen in parallel RER profiles, one with 2 stacks and one with 3.
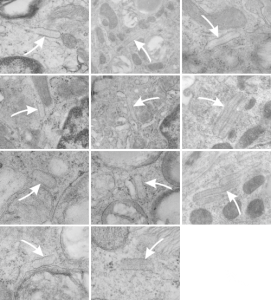 The most important observation here, aside from the more central dense line in the granules, is there apparent increased density on the limiting RER membranes, the paucity of ribosomes from the parallel edges, the presence of ribosomes on the leading edges, and a very easy to see “rigidity” to the whole granule when compared to other nearby RER profiles which have variable widths , and also NO central or multiple parallel densities within. Also, a fixed width to the type II cell granule (ICB) appears to be roughly equivalent to about 4 or 5 ribosomes (nearby on the same image) still seems to be about 100nm, and counting the periodicity in the middle and upper and lower densities along the long axis of the granule might be about 7 dots per 100 nm, which actually gives the protein within and the linear periods in both x and y axes similar dimensions: 7 bands high, 7 periods in length. One would be remiss not to mention that tubular myelin is kind of a “square tubular lattice” so there might be a connection.
The most important observation here, aside from the more central dense line in the granules, is there apparent increased density on the limiting RER membranes, the paucity of ribosomes from the parallel edges, the presence of ribosomes on the leading edges, and a very easy to see “rigidity” to the whole granule when compared to other nearby RER profiles which have variable widths , and also NO central or multiple parallel densities within. Also, a fixed width to the type II cell granule (ICB) appears to be roughly equivalent to about 4 or 5 ribosomes (nearby on the same image) still seems to be about 100nm, and counting the periodicity in the middle and upper and lower densities along the long axis of the granule might be about 7 dots per 100 nm, which actually gives the protein within and the linear periods in both x and y axes similar dimensions: 7 bands high, 7 periods in length. One would be remiss not to mention that tubular myelin is kind of a “square tubular lattice” so there might be a connection.
Rabbit alveolar type II cells: Electron microscopy – three possible RER profiles that might fit surfactant protein synthesis granules
Three possible RER profiles that might fit surfactant protein synthesis granules that have been found in other species. Publications and images found so far have not shown rabbit to have RER profiles which contain a lamellated type organization as has been seen for ferret, guinea pig and dog. It is questionable, but maybe likely, that if i had 400+ micrographs of rabbit like I do for guinea pig for dozens of separate animals, that something would show up in one or two of them. But these not-so-great electron micrographs of rabbit alveolar type II cells (from three different experimental protocols: including post artificial blood infusion) might make the suggestion that more research would be needed, unlike what I have seen in mouse, where there doesn’t seem to even be an inkling of such structures.
Long evans hooded rat alveolar type II cell: electron micrograph (more colors)
Long evans hooded rat alveolar type II cell (same as previous post) pseudocolored with most organelles identified:
Nucleus, yellow; cytoplasm of background cells and extracellular matrix, pale purple; lamellar bodies, blue; cisternae of RER, cyan; mitochondria, dark orange; cytoplasm of the alveolar type II cell, orange.
Awesome biology: exercise, health, surfactant protein A
This post is something of a revelation, I think I knew this stuff from all the last 9 months of working on Surfactant protein A (SP-A) but it was pretty much exciting to think about all the times I have recognized its function while it was still unknown to me from a scientific standpoint.
Beginning with the observation (of about 30 years duration) that I rarely got upper respiratory infections. Why? you ask. It seemed to me to be directly related to my regular practicing the flute. I am not accomplished at music, but I did practice half hour or so, and at some points more, each day, and also the timing of my practicing might be important, as that was usually just before bedtime (specifically after the kids were in bed — or at least lying on the bed where I was positioned at the foot on the floor trying to multitask… stretching the leg tendons while playing Bach. ha ha. This resistance to upper respiratory tract infections was a real phenomenon in my mind, and I have understood it to be related to playing the flute for many years.
I also remember some “old wives” medicine tips…. that sounded like this. “If you are asthmatic, learn to play the clarinet” This was observational medicine, no question of its validity.
I stopped playing the flute a few years back, but took up running (upon the challenge from my daughter (39 years my junior) to run a marathon. Then we shaved the idea down to a half marathon (which we did both complete), and I kept running more or less 5 times a week. I still didn’t get upper respiratory infections very often… maybe a little more than before, but age is a variable too. So aerobic exercise (running) took the place of my “inhale exhale” exercise from the flute.
So also, taking up the study of strange layered intracisternal protein granules in the alveolar type II cells in the lung of a couple of mammalian species has taught me much about pulmonary biology. SP-A is an awesome protein, highly conserved, and really really important for innate immune responses… helping with so many of the “wellness” functions that occur in the lung, not just what its name would suggest, being a surfactant and lowering surfact tension in the alveolar spaces. So. here is the thing….PREVENTION is the best MEDICINE — go exercise yourself, or play the flute, or clarinet, or french horn, or tuba, or trumpet, or or or…but inhale “life” deeply, stretch out that chest, breath hard, exhale in a hurry, do something aerobic. In doing so, you force those type II cells to export those lamellar bodies which contain surfactant, which includes a significant amount of SP-A). The latter then helps opsonize all those viruses and bacteria, and other stuffs that cause disease, helps bring surfactant back into the type II cells for recycling, and helps move the junk into alveolar macrophages for “removal” from the lung, modulates inflammation and antigen processing…. your whole health will improve. All that SP-A is there for a reason, exercise your lungs, use that SP-A, be well. — and it is cheaper than the MD, and safer than the Scripts, your brain will thank you too, and it is there for you BUT ONLY IF YOU EXERCISE IT.
Lung lesion: guinea pig alveolar type II cell
As promised, here is another pseudocolored electron micrograph (TEM) that I posted before, this one has all the mitochondria, and the golgi and the RER profiles separated out, in addition to the nucleus, lamellar bodies, and cytoplasm shown before.
I really wanted to add some “teeth” to this to make a joke for halloween…. I might do that, because the blue nucleus looked so much like a “skull” shape, just by chance.
But in seriousness, this lesion of a guinea pig type II cell just didnt show me any proviles of RER that had any kind of striations, layering, or indication that there was any protein organization that might be construed as what is seen in the other guinea pig alveolar type II cells shown in this quest.
Nucleus, green; lamellar bodies, blue; RER, orange; golgi, orange-red; mitochondria, turquoise blue; cytoplasm, pinkish; background and other tissue, pale peach.
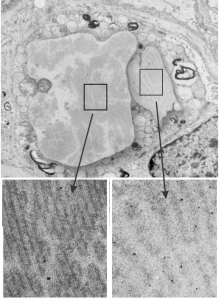
Hamster alveolar type II cell: electron micrograph
Still searching, here, for clues as to what types of organizational patterns can appear in the rough endoplasmic reticulum of alveolar type II cells. It becomes apparent that not all species have these organizational things, the intracisternal bodies, or granules. The hamster comes close, perhaps, and if this is not my imagination, I have highlighted a periodicity (mostly dotted) within a few profiles of RER in the cytoplasm of an alveolar type II pneumocyte. There are some obvious differences between this organization (if it is not artifact) and the granules that appear in the guinea pig, ferret, and dog type II cells that are noteworthy. 1) if the dotted appearance is real, then it is perpendicular to the long axis of this RER profile, and 2) if this is a surfactant protein organization at all, then it doesn’t demonstrate the same periodicity as the other three species. This doesn’t mean of course that it is not a surfactant protein, ’cause it likely is. Even at the widest part between rows of “dots” there is probably not 100 nm… maybe 80 or 90, so that puts the size a little off. Ribosomes in blue can be used as a reference to approx 20-30 nm diameter.
Yellow orange body is the RER profile with dots, purple-blue, ribosomes for comparison in size. (neg 7287 block 24716 hamster, born 9 1981, presumably female, animal 2 of three, @ 1.5 yr old.
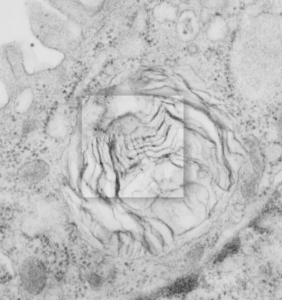
Lamellar body in an alveolar type II cell has surfactant-like-tubular-myelin grid pattern
Lamellar body in an alveolar type II cell has surfactant-like-tubular-myelin grid pattern. This was found in an electron micrograph of a ferret type II cell (neg 9872, block 23462, ferret # 7, control, 6 10 1982). This particular lamellar body is unusual in that within the lamellar structure, there is a grid like pattern which is normally seen only in the alveolar space. While, according to the literature, alveolar type II cells can internalize mature surfactant from the alveolar space, this doesnt anatomically seem very likely to me as an explanation for this particular structure. I thought it was unusual enough to post. I put a bounding box and a drop shadow around the area of interest. The grid inside has at least 4 partitions horizontally as well as vertically. Surfactant protein A is likely responsible for the grid (according to consensus) though this is usually in the alveolar space. This mineature tubular myelin was described in a book, ed Jacques Bourbon I think, which was reported to be about 1/3 the size of tubular myelin in the alveolar space.
Here are two reference images for the above micrograph to give approximate sizes. Regular tubular myelin has a side to side distance of something around 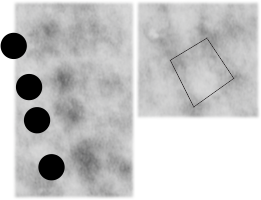 100 nm, whereas this intra-lamellar-body mineature tubular myelin sample is about 60 nm? Ribosomes are 25-30 nm (black circles)
100 nm, whereas this intra-lamellar-body mineature tubular myelin sample is about 60 nm? Ribosomes are 25-30 nm (black circles)
Alveolar type II cell RER inclusions: two different proteins? two different organizations
THis particular alveolar type II cell (from one of my most favorite guinea pig blocks, i.e. # 301, comes this profile in which there are two enormous RER bodies (at this point I don’t think it is fair to call them granules, but something else, I don’t know what) which are upwards of 20-30 microns across. One (on the right – see inset box) has the organization of a usual intracisternal body or granule that I have repeatedly found in guinea pig, at least the animals that were in our experimental group back in the 1980s) and the other does not. I am not bold enough to call this a separate surfactant protein type of granule (as opposed to what i think the right hand granule is, that is, surfactant protein A) but it certainly has its own unique banding.
Surfactant A protein and bilayers: does this in vitro data match in vivo data?
This research study uses what they call surfactant A filamentous structure and lipid bilayers to study whether surfactant protein A is responsible for some of the functional and structural features of surfactant. In one of their figures I tried to match their banding pattern with that found in vivo in the various animals I have looked at searching for an identity for this RER granule (or intracisternal body) and the numbers don’t match up completely. Their banding in vitro between surfactant protein A filaments and lipid bilayer material is more than the 100 nm periodicity I find in vivo in guinea pig and ferret and dog alveolar type II cells. I am certainly not challenging their measurements, I would more likely challenge my own, since I have not photographed a grid pattern in decades with which to calibrate the TEM. SO, that said, I conclude that there is the possibility that something in surfactant A is at work here, and the observation which is much more interesting is that they describe intersections at right angles and bran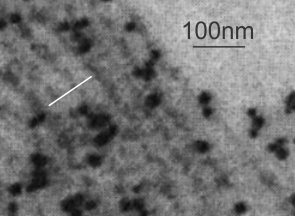 ching in vitro… just like the branching and curving that is found in the granules (intracisternal bodies) in vivo.
ching in vitro… just like the branching and curving that is found in the granules (intracisternal bodies) in vivo.
Nades Palaniyar, Ross A. Ridsdale, Stephen A. Hearn, Yew Meng Heng, F. Peter Ottensmeyer, Fred Possmayer, George Harauz American Journal of Physiology – Lung Cellular and Molecular Physiology Published 1 April 1999 Vol. 276 no. 4, L631-L641
This is a crop and edit of one of their pix. The white 100 nm bar is mine, made from their scale marker of 50 nm.