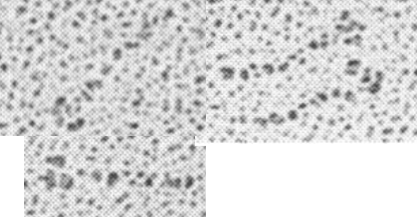
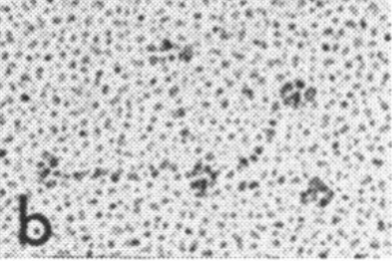
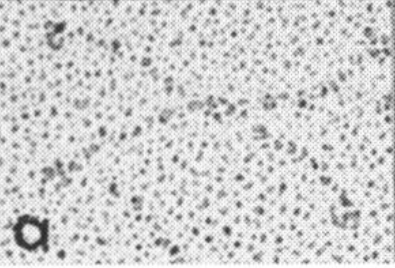
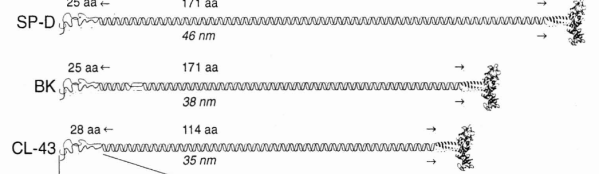
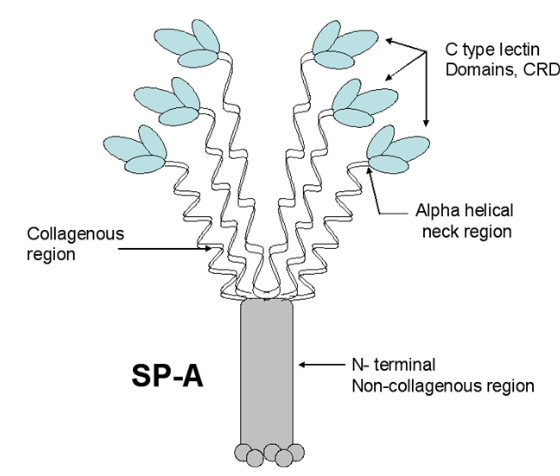
Holmskov et al published images of CL-43 and SP-D and conglutinin prepared in hopefully all in the same manner of rotary shadowing technique and magnification, and i took each of those trimeric arms measuring them separately (using a line with sufficient numbers of nodes to account for the curvature of the molecules, from the edge of the CRD to the center (presumably the N terminus). The arms of SP-D dodecamer were each measured and a mean found, similarly for conglutinin, and 4 individual arms of CL-43 taken at random from his picture (60nm, 47nm and 46nm respectively). The micron marker is hopefully accurate, and it does then show a difference in arm length with each trimer of SP-D at about 60nm (making the dodecamer about 120nm in diameter) the conglutinin arms individually at 47nm, the full diameter at 91nmn, and the CL-43 very close to the conglutinin at 46nm (no dodecamer found in his image). (you can see his figure numbers still on the unretouched images).
So a really easy way to measure the length of a curved c-type lectin arm (including bends and kinks) is to create a vector line with nodes at places which accommodate the shape (this is a different method than calculating the arc angle and arc length that I tried previously. This is quite easy by comparison. This group published a diagram with relative sizes here as well which also has micrographs accompanying, maybe some of the same images – i will check and measure. I know at one point i did not agree with the length of the collagen-like region, and had difficulty matching that with the AFM images produced by others. His diagram of the AA sequence size comparison i have posted before, but include at the bottom. THey are pretty close to what i measured. Relative to their SP-D being a single trimer of 60nm in adjusted length (adjusted for curvature) then the CL-43 is 46nm as a single trimer – exactly what his diagram would suggests (if SP-D is 60nm, then CL-43 is 47nm),
Images below