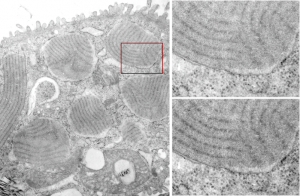
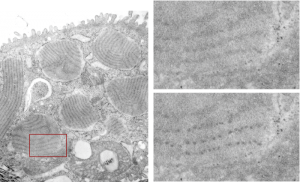
More ferret alveolar type II cell tangential periodicity in RER granules. The periodicity is easy to see. The diagram is arranged as previous post, the whole cell on the left, the red box shows the insets on the right, and upper right has the original unprocessed, and the lower right is “burned” in the areas that I think are indicative of spaced molecules (hopefully this is surfactant protein A). I am open to discussion on this.
Monthly Archives: July 2016
Ferret alveolar type II cell RER inclusions (layered protein granules)
Ferret alveolar type II cell RER inclusions (layered protein granules) appear as evenly stacked bodies, or often on tangential section, areas smeared out concentrically or linearly. These intracisternal bodies, which i think I should begin to call surfactant protein granules (ha ha, not to rile up any feathers) have many subtextures and periodicities which show up when I examine my stash of electron micrographs close up. Bless my scanner and photoshop, as they allow me to crop and highlight and darken and make what I see initially, into something that can be highlighted for everyone to see. Such is the case with several of these intracisternal protein surfactant granule bodies found in a ferret alveolar type II cell. The original cell is shown in the video, and a small inset is enlarged, and the periodicity of the protein (which I think is an accumulation of surfactant protein A — maybe upregulated in production as an immune response? I don’t know) along the dense bands of the 100 nm periodicity. The lighter bands don’t really show up very well on tangential sections, though I have some micrographs where some substructure is visible. The images used to make the video below is here as a png file, where the enhancement of the periodicity can be seen in the figure in the lower right, the area from which this inset is taken is shown in the red box on the left. The entire ferret alveolar type II cell is on the left, apical border facing top. There are at least 6 inctracisternal body surfactant A granules? present, some showing parallel substructure other showing curved and tangential cuts.
Here is a short animation using these transmission electron micrographs
Electron lucent areas just beneath the RER limiting membrane alveolar type II cell
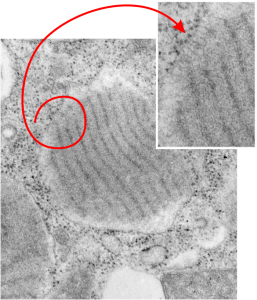
Electron lucent areas just beneath the RER limiting membrane alveolar type II cell, these have ribosomes and layered protein, just like the previous post. I suspect it has to do with the proximity of ribosomes to the protein beneath, and the lucent areas just seem not to have close-by ribosomes. The red circle highlights which part of the intracisternal body is being noted, and the arrow head points to the insert and enlargement of that arrow. As before, the original large image is unretouched while the inset has been “burned” in the area that I want you to see, which includes the more electron dense area and adjacent more lucent areas, and the ribosomes. This is an alveolar type Ii cell from a ferret, magnification is 19,500, enlargement before scanning is 4 x. Final magnification can be more or less approximated by the size of the ribosomes (reportedly 20-30 nm) which makes the dark bands within the intracisternal body separated by a distance of about 100 nm.
Electron lucent areas just beneath the RER limiting membrane
 Electron lucent areas just beneath the RER limiting membrane seem to be a feature of very large and complex intracisternal bodies (ICB) (or RER SP granules if you prefer to give them a name that is what I think they may be (that is surfactant protein granules, backlogged in the RER for some reason)) This particular ICB has all the earmarks of the same layered striations seen in guinea pig and dog (this is from ferret, and is way more abundant that typically found and more curvey as well, 6911, 23303 19,500 original mag, 4 times enlargement plus whatever playing i did in Photoshop to crop and enhance contrast).
Electron lucent areas just beneath the RER limiting membrane seem to be a feature of very large and complex intracisternal bodies (ICB) (or RER SP granules if you prefer to give them a name that is what I think they may be (that is surfactant protein granules, backlogged in the RER for some reason)) This particular ICB has all the earmarks of the same layered striations seen in guinea pig and dog (this is from ferret, and is way more abundant that typically found and more curvey as well, 6911, 23303 19,500 original mag, 4 times enlargement plus whatever playing i did in Photoshop to crop and enhance contrast).
Noted here is the pale area beneath the cisternal membrane (RER membrane) which waxes and wanes around the central layered protein area which is considerably more dense than the pale areas. And then in occasional areas where ribosomes are present on the outer edge of the membrane, there can be some streaky connections (see inset) and a little bit of increased electron density in that electron lucency. In the inset the ribosomes, 3 of them, and streaks, 3 of them, have been “burned” using photoshop… the larger and original image was not “burned” in that area and the densities can still be easily seen (red lasso around the area that is enlarged for the inset which lies at the end of the arrow tips).
Why I hate Frank Niesen Company, Cincinnati Ohio
Sweltering heat here today. I do have central air, so why not turn it on? It has been about 8 or 10 years since I used my central air with any peace of mind. It stopped working back then and I hired a company to see what was wrong. That was Frank Niesen Company, Cincinnati Ohio. In retrospect I think they flat out took advantage of the fact that I was an old lady, and they could just snow me. But anyway they said I could not have the old air conditioner fixed, that a new one (for around $2000) was required. Like a lamb, I did it without getting a second opinion.
Frank Neisen Company did put in a new compressor, Ducane, and for whatever reason, after they replaced it the weather was cool and I did not use it much, if all, for the rest of that summer. The next summer, on a hot day I turned it on, and it did not cool the house. I was angry, no question, and I called them and their response was “no longer under warranty”…. I had never used this new unit. How terrible is that. So I hired Another Heating and Airconditioning, they came out and said that whomever had installed it at Niesen had not tightened down whatever valves hold the freon. So for $200 I had to have new freon put in and I called Niesen back, and they were unresponsive.
So I used the airconditioning a few times, maybe more than a few, I think it was a pretty hot summer, then came fall and I didn’t use it again until the next year. So the first hot day I turn it on and it doesn’t cool. I called both companies. Niesen refused any responsibility, Another Heating and Air conditioning came back out and tested the Ducane condenser. A microleak they said, it has a microleak. This means that every time i use the airconditioner, it leaks freon from the added pressure I guess. I called Ducane, and they did not offer any help.
In the past few years I have only used airconditioning (it is 86 in the upstairs at the moment) becuase every time i turn it on, it blows freon out, neither nice for the environment or nice for my wallet. One should not have to deal with a company like Niesen…. they were guilty of poor installation, and also installation of defective equipment. What recourse does one have? Well. I hope this RANT makes it possible for others to see what NOT to use as a company for plumbing or air, or whatever Neisen also does. Too bad.
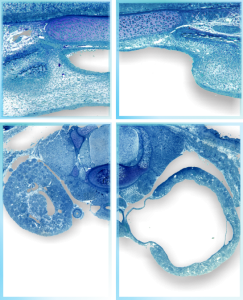
Cover submission on cleft palate and hydronephrosis
This cover submission didn’t get accepted either. Top row of micrographs represent the palate: left is control, right is cleft. The bottom row of micrographs represent hydronephrosis: left is control, right is experimental. I could look up the conditions that prevailed, but that would be “work”. ha ha.
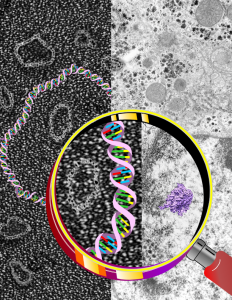
Cover submission, DNA, transmission electron micrograph, hand lens
This was a pretty outrageous cover submission which wasn’t accepted by the intended journal, but never the less it has come “curb” appeal. I don’t at this point remember which article it was to accompany, or even which journal it was submitted to. I do know it was made for a friend at UNC. And while I have no time at all…. i would be willing to make a cover for some scientist somewhere who was interested in artsie craftsie – but totally scientific illustrations.
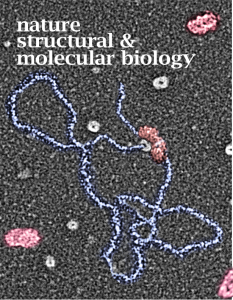
Unsuccessful cover submission 1
It is always a little heartbreaking to create a cover submission and have it end up a reject. But rejection is part and parcel for being in science and art both. Below is a fun idea I had (and a variation or two) that apparently didn’t fly with the editors (LOL). And the top image actually did make it into press. Nature structural and molecular biology, 2010, v 17, no 10.
Anatomy of a really bad diagram
It is a common problem for scientists to be unable to communicate ideas, problems, discoveries, hypotheses and conclusions to colleagues and laity. I think it is because there are so many levels of “knowing” something. Not all people can translate knowledge into pictures. That sounds like a silly statement, but personally, there are many ways that I “know” the cells and tissues that I work with – but mainly I know them “visually”, and I have many many colleagues who “know” cells in a different way, and really have no clue as to whats going on structurally, or where.
I see this disconnect when one of my colleagues says something like – “we found this in liver” or we see this in “skin” or the changes are in “lung”…. what!!! that is completely wasted talk, there is no information in those statements, at least for me. I think of liver as a mix of parenchymal cells, supporting cells and systems (venous and arterial and neurological), matrices and architectural components, secretory and excretory elements, immune and detoxication apparati, protein production, carbohydrate storage, not to speak of all the myriad and wonderful ultrastructural components of each cell type, such as hepatocytes, duct cells, endothelium, that most individuals have never seen. I could go on and on…. in all humility…. I know nothing about liver, but apparently, neither do they. There is no useful information in the word “liver”, at least to me. Saying “liver” is like saying “someday” to a person who is asking for a specific point in time to be declared.
Similarly, after many years of working on the mouse back skin models of carcinogenesis I began to dread hearing others talk about “skin”…. Skin is a complex organ system, and conservatively speaking, has a dozen unique cell types, and about as many functions known to date, and many many more unknown. This has come to be true of every organ and tissue and cell type I have looked at through the electron microscope. And just for the record, in so many ways, cell cultures just don’t function like, or look like, cells in situ, that I just cringe when the parallels are drawn. Yes, I confess, we all learn from reducing systems to their lowest common function… I understand that.
So the bottom line here is that a “careless use of words can cause confusion BUT a careless diagram causes 1000 times more confusion“. Shall I list the ways the image below causes me grief.
- The alveolar type II cell is not a box, though diagrams giving it a cuboidal shape are usually acceptable, this one is not. The alveolar type II cell (as this box doesn’t represent (below)) has distinct plasmalemmal portions which have very different functions and morphologies, e.g. the apical (microvillar) area that interfaces with the alveolar space, and the basal or plasmalemmal side adjacent to the basement membrane, and the cell-cell lateral membranes as well, none of which is pictured or identified in the diagram below, thus creating a false impression of the cell membranes. In fact the lamellar body pictured inthe diagram is positioned such that it looks closer to what I would be inclined to assume is the lateral plasmalemma, than the apical membrane, which is completely erroneous since lamellar bodies are exocytosed from — and surfactant components are recycled from — the apical membrane.
- The nucleus here is completely wrong. There are no breaks in the inner and outer nuclear membrane, nope, and the only punctuations in the nuclear envelope (composed of the inner and outer membranes and the perinuclear space) comprise nuclear pores, which makes the nuclear membrane continuous not dashed. No breaks as pictured below. Yes the perinuclear space and outer nuclear membrane are intermittently continuous with the RER, this is true, but I have never in 40 years seen a stretch of continuous RER that almost completely encircles the nucleus as alluded to in the diagram just below the nucleus… this is careless, and false information about alveolar type II cells where the RER is normally short flattened cisternae.
- It would be wonderful if when diagrams like this are prepared for publication if someone made a decision to either use 3D or 2D and stick with it. (Similar issues are seen when in writing a manuscript, the author jumps from present, to future, to past tense..ha ha) Case in point: the cylindrical objects for two transport proteins are picture in something that is supposed to be 3D (no attempt at using a structure that might actually look something like the protein they are depicting) and 2D for the remaining structures. They give prime time to the one transporter for phosphate and this erroneously gives the impression that this is the only channel, transporter, or pump that is relevant in type II cells homeostasis which is totally wrong. And even if one declares it was done for simplicity’s sake, this doesn’t hold water since the disease that a malfunction of this sodium/phosphate transporter protein might produce is quite rare (according to the authors) so to give it so much press seems silly.
- It has been my observation that the nuclei in the type II alveolar cells is more central and basal than off in the corner, so this is misinformation as well.
- The appearance of lamellar bodies can be close to the base of the cell, on the sides and also in the apex, but clearly the exit is from the apical membrane in all likelihood so the position of this lamellar body might have been better placed nearer whatever membrane portion was supposed to represent the apex. Judging by the blue arrow, pointing to the electron micrograph (which could have been much much larger for clarity) shows the lamellar body exiting the cell in the lateral membrane, which of course is wrong.
- When diagrams of lamellar bodies are made, one could quite easily approximate the “real look” of lamellar bodies, which as the name suggests contains many many lamellae….and often is as much “empty” space as lamellar space, so why for goodness sake is this lamellar body shown as a solid object? That is just missed opportunity to provide useful and accurate visual information, rather than the information that is portrayed…. which is just inaccurate.
- As for putting in the diagram the golgi apparatus, (yep i should have used a capital for the G in golgi, as identified in 1897 by Camillo Golgi, but too long ago to be a relevant decoration) as the ONLY pathway for proteins from the RER and the only pathway from golgi to multivesicular bodies is much too restrictive. Mature lamellar bodies can be found in all quadrants of the cell, though some propensity exists for larger more mature lamellar bodies to be found at the apical membrane, many are found laterally and basally, which then renders the diagram below, inaccurate once more.
- A small comment, Type II alveolar cell, vs alveolar type II cell…. a little backwards in terms of convention. and I have done it as well, but stating the organ or tissue location is a good idea before naming the cell subtypes, especially since there are other types of type II cells. In a quick google search 53 occurrences of alveolar type II cell, vs 3 (one of which is mine, sadly) of type II alveolar cell, and quite a few times, type II pneumocyte, and sometimes lung type II cell. Convention is not always good, but in this case probably would have been nice.
- The long intracellular distance the lamellar body has to traverse to exit what should have been the apical membrane (but here the exocytosis will occur on the wrong side (laterally) in this diagram and likely an inadvertent choice for the position of the arrow) is not consistent with what is seen microscopically, in addition, the electron micrograph in this diagram (the tiny black and white picture to the right of the box-cell) is tubular myelin, which in this particular instance is a very unusually large amount that is not often seen that way. This is a false indication of what surfactant actually looks like in the alveolar space of the cell fixed for fine structure…. in fact in these researcher’s own publications, surfactant with NO surfactant protein A, has NO tubular myelin at all, yet they reported no respiratory distress…. thus the image is generates false information while making a point. The scrambled looking surfactant more typically seen, with only tiny amounts of tubular myelin, is still perfectly functional surfactant, and the unorganized stuff is actually much more abundant in micrographs than the would be assumed from the tubular myelin inset below. I am not sure why one would even want to suggest that all surfactant looks like tubular myelin.
- Just to the right of the tubular myelin is a cartoon of a lipid bilayer. That diagram is most typically used to represent a cell membrane (plasmalemma) and in this case it is repeated…a large and a reduced version…. what? First of all, this lipid bilayer is not in any way what the lamellar and tubular myelin surfactant looks like. That lipid bilayer doesn’t have any proteins attached…. functional surfactant (by these authors many publications) has protein components, partly for structure partly for function, partly for other purposes (breakdown, recycling, innate immunity, others about which I do not know — but they probably do). The question is “why use this model of a cell membrane bilayer as a model of “what” do they really mean this should represent surfactant within the alveolus? There is so much misinformation in that depiction I can hardly tell what the purpose of the diagram (and its miniature friend) was.
- OK, here is an odd comment, but what was the purpose of the red arrow from the alveolar space back to the multivesicular body? And what was the purpose of the word “catabolism” — just silly redundancy. Who in life doesn’t understand the word recycling? Doesn’t that suggest taking apart and repurposing, AND to suggest that all surfactant is recycled through the multivesicular body…. yikes, there is a real leap, that cannot be substantiated by any electron micrographs of alveolar type II cells I have ever seen. I will have to check the position of multivesicular bodies (I won’t take time to quantify any results or create a dataset, but just from my observations, MVB are neither sufficiently frequent, nor sufficiently large to accommodate the volume of turnover, though they repurposing of some components of the uptake of surfactant from the alveolar space might well get shuttled off to MVB in those low amounts.
- So if the Abca3 transporter moves DPPC into lamellar bodies, this diagram suggests that it does so from the cytoplasm (but I think that this is moved through the membranes. OK, more later.
- OK, so i am bored of this now, I am certainly grateful that they didn’t deem it necessary to add the diagram of an alveolar macrophage. That might have triggered another rant. Best to all these authors, each of whom I have met. But this was a great exercise for me, and a wakeup call to all you scientists who think that you can carelessly slap together a box and a few ovals and a label or two and do any one any favor. This was clearly only accepted for publication by reputation, not by information.
Odd protein accumulation within type II cell of an aged guinea pig
Ultrastructure shown here of more unusual protein structures within the RER of type II alveolar cells, these are really not at all like what I have been thinking are SP-A, but are more like fuzzy balls. The dimensions of the rounded electron dense accumulations within the cisternae of RER within this type II cell are about the dimension of 100+ nm. The comparison in size between three ribosomes from this same image (see the inset in lower right at actual size) and three of the fuzzy balls? perhaps, in the same inset at actual size, and then see the two types of structures, i.e. the ribosomes and the fuzzy balls enlarged to the same degree and pseudocolored in the inset to compare their relative sizes. The ribosomes are supposed to be something around 20-25-30 nm in that range, and if these are fuzzy balls (that would be multimers of surfactant protein D) then the fuzzy balls should be about three diameters compared to the ribosomes. Doesn’t quite work out, so the identification of these electron dense objects within the cisternae of RER in this electron micrograph is up for grabs. Nucleus is pseudocolored blue, nuclear pore, green, RER profiles with the odd protein, orange, actual size ribosomes and fuzzy balls grey, in inset, and enlarged in relative size, ribosomes, blue, fuzzy balls red.