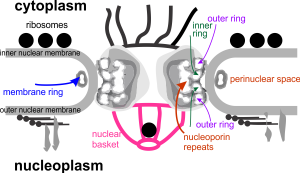
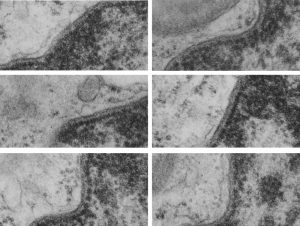
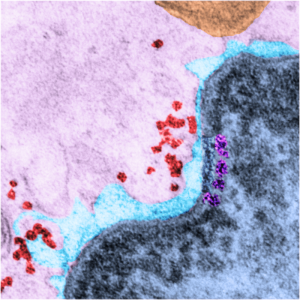
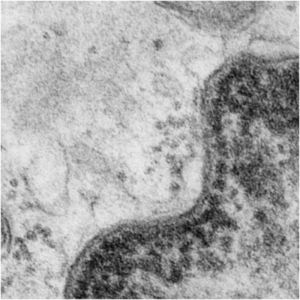
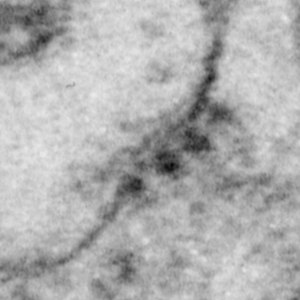
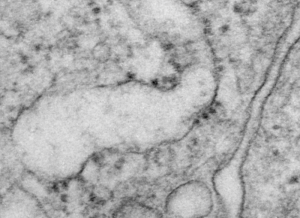
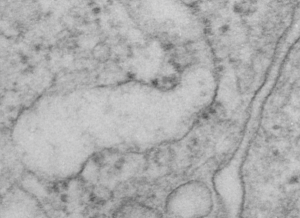
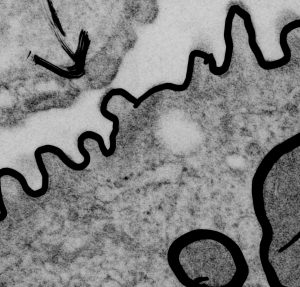
Yesterday’s post on a nuclear pore from a type II alveolar cell i pursued a little more, trying to establish a relative size for the current image and models of the nuclear pore complex, and a website posted this terrific image which i made transparent and placed into the transmission electron micrograph of of a nuclear pore from my studies. There are some issues using their image which is a top down view, and my image which is sectioned more from the bottom up, but it is an interesting match. One thing i like about this particular model as opposed to many of the diagrams seen online is that it does NOT extend the nuclear basket too far into the nucleoplasm and have the inner and outer nuclear membranes too spaced apart in the area just adjacent to actual pore. It appears in my micrographs as if the cytoplasmic filaments are greater length than any basket structure seen on the nucleoplasmic side. So this more compressed model (fading in and out of my micrograph below) works best.
website where i found the image of the nuclear pore is here: http://phys.org/news/2015-06-high-performance-microscope-pores-cell-nucleus.html#nRlv