Looking at lysosomes in the lung of mice which have breathed perfluorochemical (PFC) liquids is interesting. These are old archived micrographs from the early 1970s but have a lot of valuable information about the reactivity of perfluorochemicals in the body (the latter being touted as totally inert. Regardless of what science says about the reactivity of PFC they do cause a mountain of effects in humans, not the least of which is an immediate uptake by the reticuloendothelial system, massive sequestering and dumping of enzymes into the resultant lysosomes, and a kind of partitioning, or re-partitioning, or re-emulsification of the PFC, depending upon whether it was breated as a neat liquid, or infused as an emulsion with other agents accompanying it.
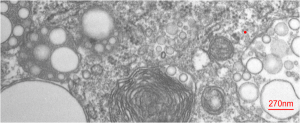
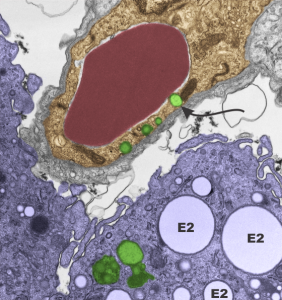
So “case in point” is this alveolar macrophage, picture taken from the lung of a mouse which breathed E2 for 3 hours and was allowed to recover for 48 hours then lungs taken for electron microscopy (and probably chemical analysis, though I have no clue where those data are at this point 40 years down the pike). I just know that my negative number is 1351, and that block number is 4840, magnification was 5,000 (Zeiss 10) date 2 17 1975 at 60kv. Mouse weighed 16.85g, breathed E2 for 3 hours on 2 3 1975, and was processed on 2 5 1975, which equates to 48 hour recovery. The sample of lung was taken from the R, mid lobe.
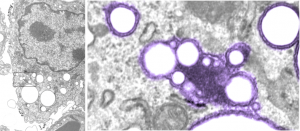
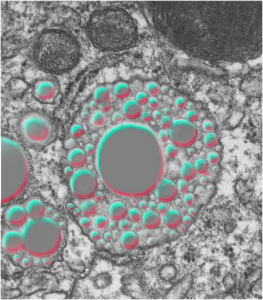
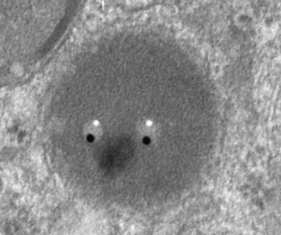
The image below (L) is a portion of the original negative (scanned as a transparency from the film) with an enlargement (box demarcates it) on the right where I have “pseudocolored” the protein densities with purple, which surround the empty footprints where the PFC was fixed (leaving the nice round spaces) in the cytoplasm. About 6 ribosomes (@27nm diameter) are also highlighted beneath the central phagolysosome?lysosome?membrane structure to show that the size of the smallest perfluorochemical droplets is in the nanometer range, before — before what, perhaps being small enough to diffuse back into the alveolar air space and be exhaled. I don’t believe anyone knows whether the larger or the smaller E2 droplets are more apt to move into the alveolar air space. There is one tiny droplet which is about 27 nm nesteled between two ribosomes… go figure. The enzyme response here is pretty amazing, and appears in section to be most often seen as spheres, but is more likely to be a longer sausage shaped lumpy body.
The bottom line of this image is that these droplets appear to migrate along the membrane systems.