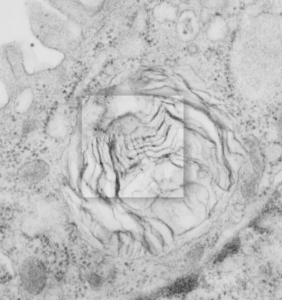
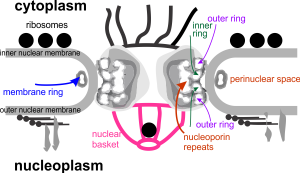
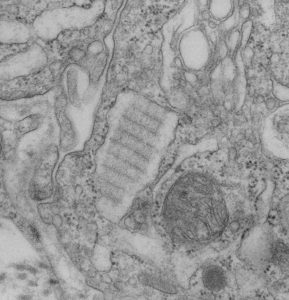
Still searching, here, for clues as to what types of organizational patterns can appear in the rough endoplasmic reticulum of alveolar type II cells. It becomes apparent that not all species have these organizational things, the intracisternal bodies, or granules. The hamster comes close, perhaps, and if this is not my imagination, I have highlighted a periodicity (mostly dotted) within a few profiles of RER in the cytoplasm of an alveolar type II pneumocyte. There are some obvious differences between this organization (if it is not artifact) and the granules that appear in the guinea pig, ferret, and dog type II cells that are noteworthy. 1) if the dotted appearance is real, then it is perpendicular to the long axis of this RER profile, and 2) if this is a surfactant protein organization at all, then it doesn’t demonstrate the same periodicity as the other three species. This doesn’t mean of course that it is not a surfactant protein, ’cause it likely is. Even at the widest part between rows of “dots” there is probably not 100 nm… maybe 80 or 90, so that puts the size a little off. Ribosomes in blue can be used as a reference to approx 20-30 nm diameter.
Yellow orange body is the RER profile with dots, purple-blue, ribosomes for comparison in size. (neg 7287 block 24716 hamster, born 9 1981, presumably female, animal 2 of three, @ 1.5 yr old.