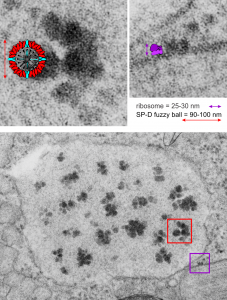
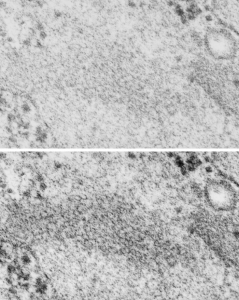
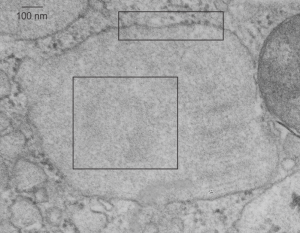
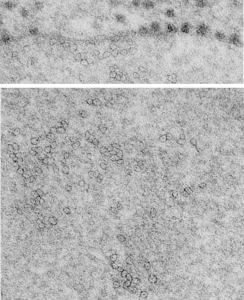
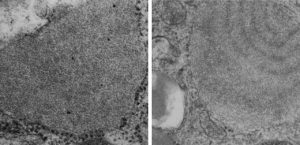
It is so fun to try to figure out whether the accumulations of layered protein in the RER in type II alveolar cells are, in fact, surfactant protein A. These images have sat on my office shelf for 30 years. As posted a few days ago, this particular electron micrograph has intracisternal bodies or granules or RER accumulation of a layered protein (call it what you want) that were common to guinea pig type II cells, but just one profile of RER had funny round “fuzzy balls” in it. I only saw this once. From the beginning it was easy to see that they were not at all like ribosomes, which one might quickly think could get stuck in the bands of such a wild production of a protein, but they are the wrong size, and have a totally different texture appearance, so ribosomes are pretty much “out of the question” as a choice. The next easiest conclusion was that they were glycogen, also electron dense, but I am not sure why there would all of a sudden be glycogen in these protein (- whatever protein it turns out to be) accumulations, and besides, glycogen is more random in its clumped appearance and is bound within membranes (one can google pictures of glycogen in the liver and see the difference). But I remembered reading about these wacky “fuzzy balls” probably composed of surfactant protein D (Patrick Waters et al, Chapter 6, in: Target Pattern Recognition in Innate Immunity, ed. Uday Kishore, Landes Bioscience and Springer Science+Business Media, 2009) and did some images tests to determine if these could be surfactant protein D. Fuzzy balls are the cruciform surfactant D, becomes a multimer – 4 crucifers in the same 100 nm (others say more like 90 nm) structures.s
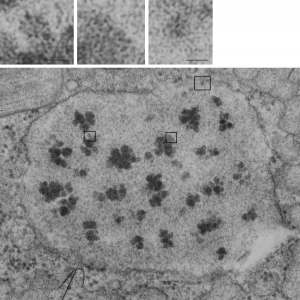
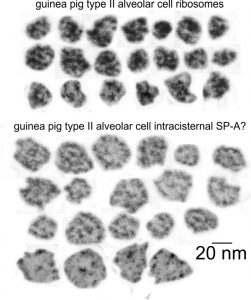
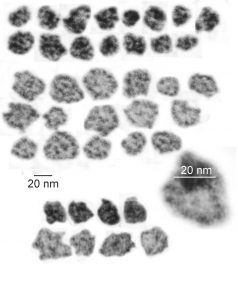
I just cropped out ribosomes from the identical electron micrograph as I cropped out a sample of these fuzzy round structures and compared their size, side by side. You see that this doesn’t come out exactly right. The proposed size for the surfactant protein D fuzzy balls is just a little bit too large, to fit nicely into the shape of what appears in my micrographs. In addition, I was hoping that the only protein found in these intracisternal bodies was surfactant protein A, and not containing any other surfactant protein. But the thought was intriguing. I do not know of any reports that indicate surfactant protein A can form similar fuzzy balls, but it seems that it might be possible…. and the dimensions for a surfactant protein A fuzzy ball would be closer to the size estimated for these, per the adjacent ribosomes.
Anyway, check out the diagrams of a surfactant D fuzzy ball and a ribosome, matched with their nm markers, and the micrograph of the intracisternal body from which the fuzzy balls were derived. If you have any interest, or information, I would love to hear about it. Red rectangle on bottom image is the portion of the fuzzy balls used, while purple rectangle on the bottom image shows where ribosomes were used.