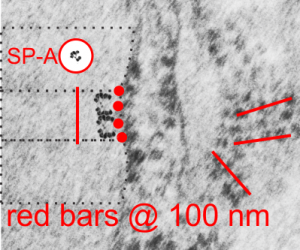
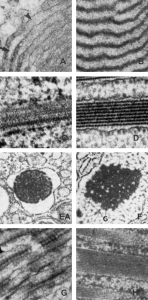
I revisited the protein acumulation in the alveolar type II cell of a rabbit (this particular rabbit was 79 days post infusion of an emulsion of oxydecalin 10% and F68 25% at 60cc/kg intravenously, on 9 9 1987. negative 11320, block 31750, and this is one of only two locations where there was a stacked (or single) profile of RER which had some of the characteristics of an intracisternal protein that might be made up of surfactant protein, in particular surfactant protein A. The criteria met include: 1) stiffness of the portion of the RER which has the protein inclusion visible, 2) loss of ribosomes on the longitudinal axes, but ribosomes on the growing end(s), 3) some evidence of layering within the granule (see inset where i highlighted what i think is evidence of layering) 4) a parallel stacking. I have considered this an equivocal demonstration of a surfactant protein granule in the alveolar type II cell, since the layering is really not that prominent. The bigger issue is that, if one considers the ribosome of the rabbit, to be similar in size to other mammalian ribosomes (I have taken as an average about 27 nm), then the width of the profile at its narrowest dimension is about 1.5 times wider than the layered granules seen in guinea pig, ferret and dog. So this is an issue. See for yourself in the image below. (two prominent scratches stamped out using photoshop, contrast increased also, otherwise no processing). Red dots=ribosomes, dotted boxes show areas of inset. Insets show areas of possible banding which I highlighted with the burn tool in photoshop. So original on right, emphasized on left.

While you are on this page, check out the awesome glycocalyx on the apical plasmalemma folds and projections in the upper right corner of the electron micrograph.