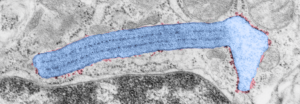
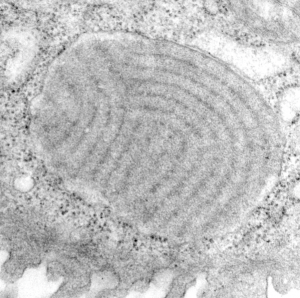
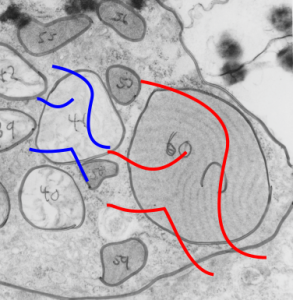
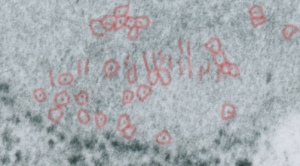Vertical and middle band banding in alveolar type II cell surfactant protein granules is shown here (hopefully this is SP-A). Using the same image as posted as the quintessential surfactant protein granule in alveolar type II cells, the image has been copied, inverted, and offset just a few pixels, to cast a shadow and give dimension to the “edges” in the image, a kind of embossing technique.
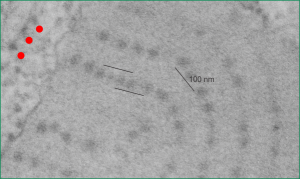
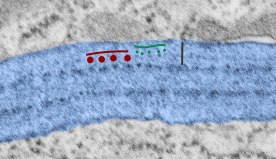
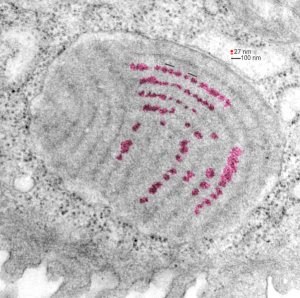
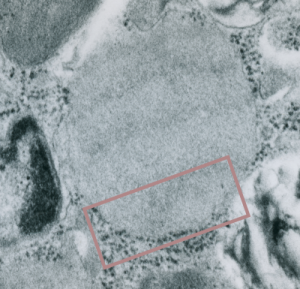
Embossing vertically highlights the bumpiness of ribosomes and other protein “lumps” within the granule, while embossing by moving the inverted image below, causes the ribosomes to appear to be concave. Both accentuate different features (see below). The embossing upward as well as moving the inverted image a few pixels to the left highlight the banding seen within the granule which is secondary to the electron dense bands that run parallel to the long dimension of the granule… the vertical lines are not present in the surrounding cytoplasm, thereby indicating that the vertically lines within the granule are not section artifact or fixation artifact but actually a part of the specific alignment of the granule proteins. The third image is a flattened image, cropped, increased in brightness and contrast, then vertical lines were superimposed where I saw definition. Ribosomes, as an internal control for magnification are about the size of the red marker (27 nm estimated diameter), black line of 300 nm = the height of the three banding periods comprising this particular granule, 100 nm bars mark out the middle dense layer (with the blue dots where periodicity shows up) and also shows that about 4-5 protein densities occur in the middle layer of each 100 nm period.
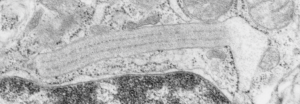
Original ferret alveolar type II cell RER granule electron micrograph, untouched, from which these processed images were derived can be found here.
Negative 9879 block 23494 ferret # 16, untreated. Electron micrograph of an alveolar type II cell with a surfactant protein granule (which in some ways is similar to other collectin, c-type lectin, style granules, where organization of these proteins is highly oligomerized–e,g, Birbeck granules). Embossed upward, and down ward images below. Bottom image is a cropped and enlarged from middle image, showing vertical lines which may be 4 or 5 per 100 nm and can transcend all three periods of this granule; ribosomes (as a size marker of @27 nm); periodicity of the medium dense layer of this granule (blue dots and 100 nm bar marker).