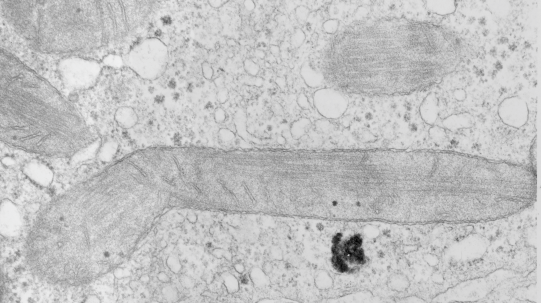
Nice tubular cristi in this hepatocyte from a rhesus monkey.
Daily Archives: December 13, 2017
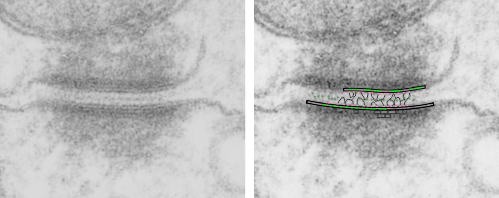
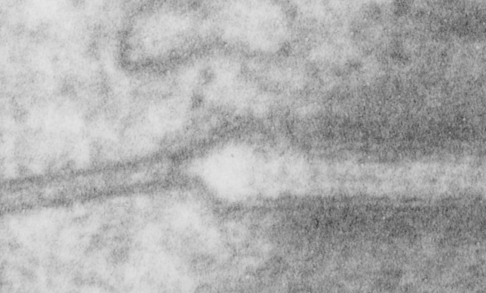
Begin here: “empty annulus” outer ring around the desmosomal protein core”
How funny this is, the perimeter of a desmosome with this very precise and quite abrupt “beginning”. It seems that adjacent cells (with a concentric area of pretty well adhered plasmalemmae (two adjacent cells involved)) make the abrupt 45 degree bend just to (coincident with?) the widened intercellular space at the core of the desmosome to its typical width of about 25nm (up from the space between two cells at its closest… more around 17nm).
What is most interesting is what signals the 45 degree spread (bend) on the plasmalemma just before the desmosomal disk, and why is it so electron lucent? or free or proteins?. This is a “free” zone before the electron dense desmosomal complex proteins that make their junctional adherent weld of about 150nm. the width of the annulus. The area of the annulus would vary depending upon where in the spot desmosome the section happened to fall, and a cut in the annulus completely would just look like a wider two-cell junction, showing no desmosome at all. Whether the width of the annulus around the desmosome is consistent from one species, or even one cell type to another is likely not known. I will google to see.
Stub tail monkey hepatocyte desomosomal-mitochondrial tether: more
There are several nice things about this micrograph of a desmosomal mitochondrial tether (i.e. tethered via the intermediate filaments of the desmosome). One nice thing is the very rigid, and parallel membrane-leaflet quality of the plasmalemma where there is the desmosomal protein complex, and it also exhibits a very even and well defined separation of the outer and inner leafelets of the plasmalemma (at least this is what i think is shown in the micrograph below). I have marked the two leaflets on the cell membranes on the adjacent cells with black lines. There are obvious periodicities within the inner and outer membrane leaflets too… not withstanding the density of the lead citrate and uranyl acetate stains and the quality of fixation) which seems to present overall patterning in the entire micrograph. I have marked the extracellular lines (which are not likely to be random as they appear, but more likely to be organized attached wishbone shapes (mirrored vertically and copied horizontally) shown in an earlier post but the lines here have been drown over actual densities in the extracellular (intercellular) space of the desmosome. Green blobs mark periodicities within the two leaflets of the plasmalemma, and the pink blobs mark the densities which are extracellular but likely adjacent, maybe even part of (like the cadherins in desmosomes) those proteins. (green blobs are INTERcellular, the pink blobs are within the plasmalemma (transmembrane). Electron micrograph on left is unretouched, on right, contrast and brightness enhanced and vector overlays show densities which can be matched to image on the left. This stub-tail monkey hepatocyte came from an animal that received two test doses of perfluorodecalin (a very small amount) a year prior, which in my experience has nothing to do with these tethers. Shown immediately below the outer leaflet of the plasmalemma is what appears to be a regular compartmentalization (shown as rectangles) likely representing a highly structure arrangement of plakophilin and plakglobin and maybe some desmoplakin and a bit of vimentin. The number of densities (green or pink, and likely connected parts of the cadherin dimers, turn out in this micrograph to be about 9 nm… a little close than found in mouse micrographs so far.