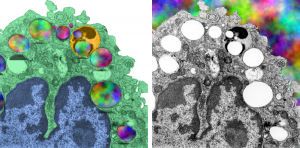
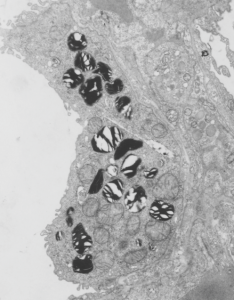
Alveolar macrophage from a mouse which was submerged in perfluorochemical (PFC) liquid and which breathed that oxygenated fluid for three hours. The mouse was allowed to recover for 17 days, then the lung was preserved for various chemical assays and for transmission electron microscopy. This experiment took place on 2-1-1975 in the laboratory of Dr. Leland Clark, Jr, who was investigating perfluorochemical liquids as possible remedies for respiratory distress and for alleviating bends in deep sea divers. This original micrograph, 10,000 x at the microscope, enlarged 4 times is my neg. 1436, block 4820. The original micrograph can be scanned and sent to any one who desires it. The most obvious anatomical markers are the nucleus, which looks a little like a pac-man mouth (LOL) and it has an active nucleolus, a few not so opportunely sectioned nuclear pores, some golgi vesicles in the cleft, occasional profiles of RER, about a dozen mitochondria are present as well. There are two structures which are NOT likely to be inclusions of E2, since they have other lamellar and whispy electron dense contents. Those circular droplets which I believe represent the footprints of E2 are colored (left) and left original color (right) in a contrast enhanced but otherwise unaltered micrograph.
There is a dense body (actually 2 dense bodies) which have E2 inclusions, the presence of PFC droplets within membrane bound lysosomal bodies accompanied by dense enzymes was common for just about every pfc that was examined, though the formation of those lysosomes (dense bodies) took several days to weeks to form. There is really nothing too remarkable about this alveolar macrophage (as far as I can tell) except for the presence of PFC. Of course I had fun with colors here, nucleus, blue; one dense body/lysosome, orange; alveolar macrophage cytoplasm, green, in left image. Right image, contrast enhanced original.