How is it that we cannot learn from our mistakes, as a society. This little compound, bisphenol A, has been a mainstay of the economy for several decades but should it be protected? just because of its massive presence, or should we learn from our mistakes, like lead in gasoline, asbestos as a fire-retardant, believing that pesticides are a good measure for crop increase, and that plastics are inert.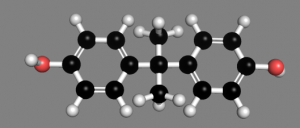
Consider the massive amount of evidence that was required before people who smoked actually believed that smoking was hazardous to their health. Consider too, the massive numbers of individuals that think that alcohol (because of its delayed onset of visible effects) is a safe and fun way to get a buzz. Consider, as well, that just because we don’t see blatant effects from absorbing the plasticizers in our every day plastic existence that they have no effect either.
HERE is verbatim what NIH – tox town has to say:

Bisphenol A (BPA) is used to make lightweight, hard plastics.
What is bisphenol A?
Bisphenol A, also called BPA, is a chemical that has been used since the 1960s to make lightweight, hard plastics and epoxy resins. In its pure form, it is a white solid with a medicinal odor. More than a billion pounds of BPA are produced in the United States every year.
BPA plastic products are usually clear and hard and are often called polycarbonate plastics. BPA plastic products include food and drink packaging, water bottles, infant and baby bottles, infant feeding cups, reusable cups, compact discs, automobile parts, impact-resistant safety equipment, plastic dinnerware, eyeglass lenses, toys, and medical devices. BPA epoxy resins are used as lacquers to coat metal products such as the inside lining of metal food cans, bottle tops, wine vat linings, floorings, paints, and water supply pipes. Some flame retardants, dental sealants, and dental composites may also contain BPA. BPA is used in the recycling of thermal paper, such as receipts, self-adhesive labels, and fax paper. It is also used to make polyvinyl chloride plastics.
Plastic products made with BPA will have a #7 recycling symbol on them or contain the letters “PC” near the recycling symbol. BPA is not one of the phthalates, which are found in soft plastics.
How might I be exposed to bisphenol A?
Human exposure to bisphenol A is widespread, according to the National Toxicology Program. Most human exposure to BPA comes through food and beverages. You can also be exposed through air, dust, and water. BPA can leach into food from the linings of canned foods and from plastic products that are made with BPA. More BPA leaches from food and beverage containers if those foods and liquids are hot or boiling. If food containers or bottles are scratched or damaged, more BPA may be released into the food or liquid. The highest estimated exposures to BPA occur in infants and children, according to the National Toxicology Program.
At home, you and your family can be exposed to BPA if you use plastic food containers, canned foods, water or baby bottles, plastic dinnerware, reusable cups, and other consumer products that are made with BPA. You can have short-term exposure to BPA following the application of certain dental sealants that are made with BPA materials.
At work, you can be exposed to BPA by inhaling it or having skin contact with it if you work at a facility that manufactures BPA or products that are made with it.
How can bisphenol A affect my health?
Bisphenol A is an endocrine disruptor, which is a chemical that may interfere with the production or activity of hormones in the human endocrine system. According to the World Health Organization’s International Programme on Chemical Safety, there is still uncertainty about some links between human health effects and exposure to endocrine disruptors.
Both the U.S. Food and Drug Administration (FDA) and the National Toxicology Program (NTP) have “some concern” for effects on the brain, behavior, and prostate gland in fetuses, infants, and children at current human exposures to BPA. The NTP has “minimal concern” for effects on the mammary gland and an earlier age for puberty for females, in fetuses, infants, and children at current human exposures to BPA. The possibility that BPA may alter human development cannot be dismissed, according to the NTP. In cooperation with the NTP, FDA is carrying out in-depth studies to answer key questions and clarify uncertainties about the risks of BPA.
In the workplace, exposure to BPA dust may irritate the eyes, make skin sensitive, and cause dermatitis, irritated skin, and eczema. Contact with BPA may burn the eyes, lips, and skin. Inhaling BPA can irritate the nose and throat and cause coughing and wheezing. Exposure can cause headache, nausea, abdominal pain, and vomiting.
More research about BPA is needed to understand exactly how current findings relate to human health and development, according to the U.S. Food and Drug Administration and the National Toxicology Program.
If you think your health has been affected by exposure to BPA, contact your health care professional.
For poisoning emergencies or questions about possible poisons, contact your local poison control center at .
This description is based on the information found in the Web links listed with this topic.
You can look up their links if you like. In the list above they forgot to mention kitchen supply lines !!!!!
MY NEW KITCHEN WATER SUPPLY LINES ARE from Brasscraft (though touted as phthalate free) are braided metal LINED WITH flexible PVC… they will increasingly (with age) give off BPA in my drinking water, more from the hot water tap than the cold water tap, but from both. If i already to not eat canned foods (lined with flexible PVC containing BPA, why would I exponentially increase the amount of BPA i consume every day by putting this in my own kitchen plumbing.
When investigating the metallic taste in the water I have coming out of my new Moen kitchen faucet I began investigating the source, and trying to eliminate it, and I found out many things about current plumbing fixtures, lines, valves, cartridges, and dopes that I really did not like.
For starters, I had copper, now i cannot replace it, why?? no longer made in 3/8 inch. I must either use all PVC flexible line, or braided metal lined with PVC or brass. I must now investigate what is going to be leaching out of the brass lines…. I know we can’t always know everything, its a huge task to even take in the changes we must, but I am really overwhelmed when I ask Moen, or Home Depot, or Brasscraft, or the plumber and they all say, well, I smoke, and I drink, and i have flexible woven supply lines with PVC, and i am just fine. ha ha…
 I appreciate this site (linked below) and its comments on BPA, as it sort of sums up what I have read about bisphenol A over the last 6 years. When we find something so entrenched in daily life as plastic might not be “good for us” it takes a huge amount of effort to effect small changes to eliminate it. This means change on the consumer side (comfort and convenience) which is hard to deny, and voluntary change on the side of the producers (profit and greed) which is very unlikely to happen.
I appreciate this site (linked below) and its comments on BPA, as it sort of sums up what I have read about bisphenol A over the last 6 years. When we find something so entrenched in daily life as plastic might not be “good for us” it takes a huge amount of effort to effect small changes to eliminate it. This means change on the consumer side (comfort and convenience) which is hard to deny, and voluntary change on the side of the producers (profit and greed) which is very unlikely to happen.